Top 6 Notorious Greenhouse Gases
Concerns about greenhouse gases are prevalent while discussing global warming. The thermal energy emitted by the Earth's surface is absorbed by these gases, ... read more...which then radiate it back to the ground. By doing so, they support the greenhouse effect, which prevents the earth from completely losing its surface heat at night. The amount of heat absorbed by the atmosphere and radiated back to the surface depends on the concentrations of different greenhouse gases in the atmosphere. Here, the notorious greenhouse gases are listed.
-
Carbon dioxide (CO2) is the most notorious greenhouse gases. Volcanoes, the burning and decomposition of organic materials, aerobic (oxygen-using) animal respiration, the burning of fossil fuels, the clearing of land, and human cement manufacturing are some of the sources of atmospheric CO2. These sources are typically counterbalanced by a collection of "sinks"-a collection of physical, chemical, or biological processes-that function to remove CO2 from the atmosphere. An essential natural sink is plant life, which absorbs CO2 during the process of photosynthesis. Marine life in the oceans can take up dissolved carbon dioxide, and some species even utilise it to create calcium carbonate skeletons (CaCO3) and other structures.
The observed rise in average global temperature and ocean acidification are due to an increase in the absorption and emission of infrared radiation by the atmosphere, which is caused by increases in atmospheric concentrations of CO2 and other long-lived greenhouse gases like methane, nitrous oxide, and ozone. The CO2 fertilization impact is an additional direct effect. Numerous indirect consequences of climate change on the natural environment, ecosystems, and human cultures are brought on by these changes. In terms of global warming, carbon dioxide has a greater impact than all other greenhouse gases combined.
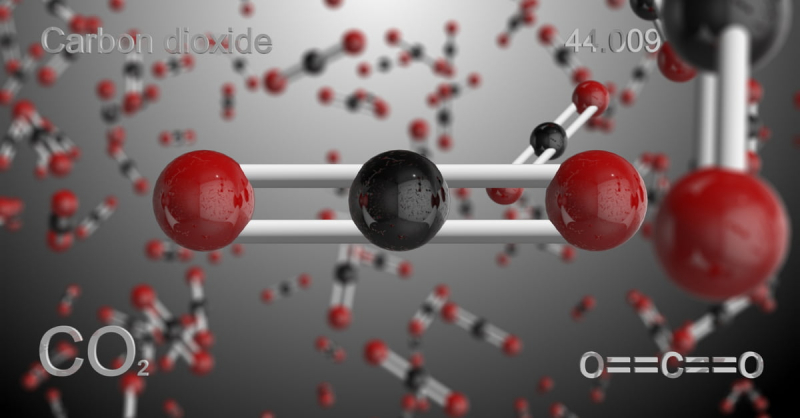
Photo: Monkey 
Photo: Phys.org -
The second-most significant greenhouse gas is methane (CH4). Despite having much lower atmospheric quantities than CO2, it is more powerful. Additionally, CH4 stays in the atmosphere for a shorter period of time than CO2-roughly 10 years as opposed to hundreds of years. Numerous wetlands, methane-oxidizing bacteria that consume the organic matter that termites eat, volcanoes, seepage vents of the seafloor in areas rich in organic sediment, and methane hydrates trapped along the continental shelves of the oceans and in polar permafrost are all examples of natural sources of methane. The atmosphere itself serves as the main natural methane sink, while bacteria in soil also act as a natural methane sink there.
Similar to CO2, human activity is raising the concentration of CH4 faster than natural sinks can reduce it. Currently, around 70% of total yearly emissions are attributed to human sources (including rice cultivation, cattle husbandry, the burning of coal and natural gas, biomass combustion, and decomposition in landfills), which has resulted in significant concentration increases over time.
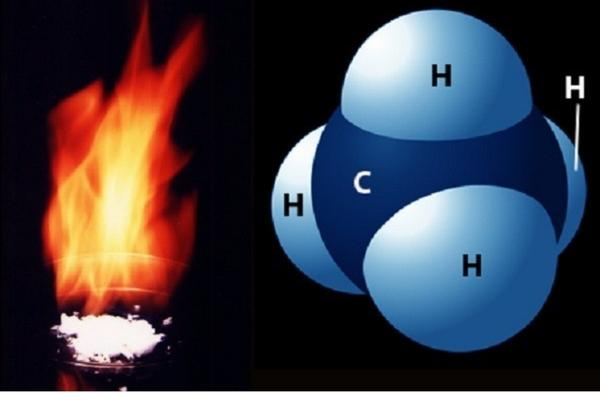
Photo: LinkedIn 
Photo: VietChem -
Surface, or low-level, ozone is the thirth-largest greenhouse gas (O3). Now that we have a gas, it is important to separate it from naturally occurring stratospheric O3, which plays a totally different role in the planetary radiation balance. This gas is a direct outcome of air pollution on the surface. The sinking of stratospheric O3 from the upper atmosphere toward the surface of the Earth is the main natural source of surface O3. In contrast, photochemical processes involving carbon monoxide (CO), such as those that occur in smog, are the main human-driven source of surface O3.
The primary photochemical reactions with or involving harmful Carbon Monoxide are the source of this gas. However, unlike the aforementioned well-known greenhouse gases, this gas has no normal option for falling, making it one of the most dangerous gases present near the earth's surface. Ozone is also the most major low-level or surface greenhouse gas.
The best estimates place the natural surface O3 content at 10 ppb, while the net radiative forcing brought on by anthropogenic surface O3 emissions is roughly 0.35 watts per square meter. In cities that are prone to photochemical smog, ozone concentrations can approach harmful levels (conditions when concentrations meet or exceed 70 ppb for eight hours or longer).
Video: Paul Cochrane 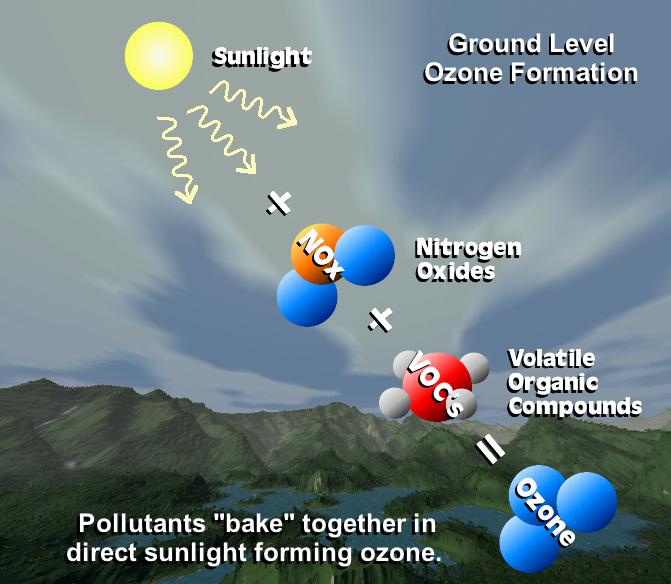
Photo: www.geo.sunysb.edu -
If you are knowledgeable about notorious greenhouse gases, you must be aware of the gases that are the most harmful, primarily nitrous oxide and halocarbons. The latter consists of hydrofluorocarbons (HFCs), perfluorocarbons, and sulfur hexafluoride (PFCs). Because of normal biological processes in soil and water, nitrous oxides have low background quantities, but fluorinated gases are mostly dependent on industrial sources.
These gases are frequently created in industrial settings and processes. Smidges of nitrous oxide can be found in the water, soil, and environment, whereas halocarbons are a byproduct of industrial activities. These two well-known greenhouse gases are among the most deadly and have the power to quickly upset the entire ecological balance of society. We also make these fluorinated gases in our refrigerators and air conditioners

Photo: Phys.org - Nitrous oxide emissions pose an increasing climate threat 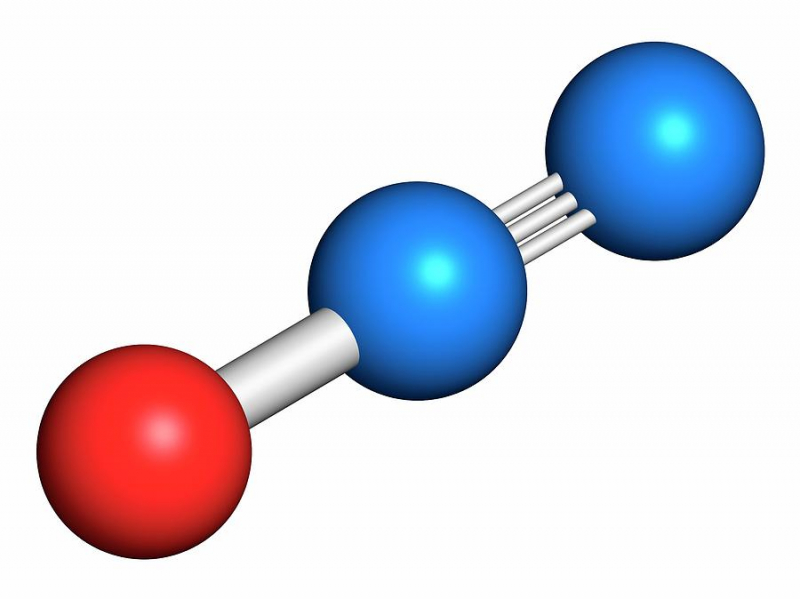
Photo: Fine Art America - Nitrous Oxide -
The most potent greenhouse gas in the Earth's atmosphere is water vapor. It sort of stands apart from other greenhouse gases. Due to the fact that human conduct cannot, in general, directly change the amount of water vapor in the atmosphere. However, air temps are what correctly set it. The rate at which water evaporates from the Earth's surface increases in proportion to Earth's surface temperature throughout the summer. As a result, increased evaporation indicates that there is more water vapor present in the lower atmosphere. It also has the capacity to absorb infrared radiation and release it surface-down.
Every planet in the Solar System, as well as many celestial objects like comets, huge asteroids, and even the solar atmosphere, all contain water vapor. The discovery of extrasolar water vapor would also suggest that additional planetary systems have a similar distribution. In the case of some planetary mass objects, the presence of water vapor can also serve as a subliminal indicator of the existence of extraterrestrial liquid water.

Photo: Wikipedia' 
Photo: Wonderopolis -
The inorganic compound sulfuryl fluoride, has the formula SO2F2. With characteristics more akin to sulfur hexafluoride than sulfuryl chloride, it is a readily condensed gas that resists hydrolysis even at temperatures up to 150 °C. Although it is a strong greenhouse gas and neurotoxin, it is frequently used as a fumigant pesticide to control termites.
Sulfuryl fluoride is dangerous when inhaled since it can cause convulsions, lung irritation, pulmonary edema, nausea, abdominal pain, central nervous system depression, numbness in the extremities, and death. These excessive exposures happened as a result of unauthorized entry into buildings during fumigation or inadequate aeration. Epidemiological research revealed neurological impacts in sulfuryl fluoride-using fumigation employees, including impaired cognitive and pattern memory as well as impaired olfactory function.
In 1987, a senior couple who had already approved their home for reentry was exposed to sulfuryl fluoride. The level of sulfuryl fluoride was not measured when the fumigation company opened windows and doors and aerated the house using fans. When the air was sampled 12 days following aeration, it was not found. That nightfall, the pair felt weak, queasy, and out of breath. The man had a seizure and passed on the next day. With pulmonary edema, his wife's condition deteriorated, and six days later, she suffered a cardiovascular arrest and passed away.

Photo: iStock - Farm Worker Fumigating With Pesticide Lemon Trees 
Photo: American Chemical Society













