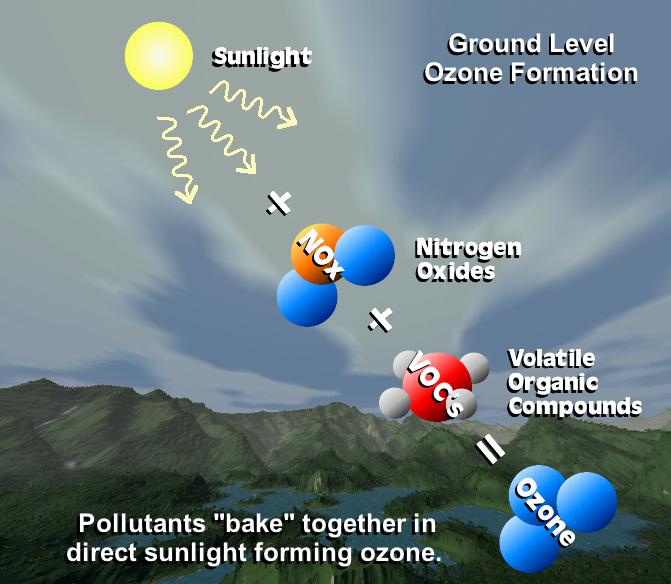
Surface-level ozone (O3)

Surface, or low-level, ozone is the thirth-largest greenhouse gas (O3). Now that we have a gas, it is important to separate it from naturally occurring stratospheric O3, which plays a totally different role in the planetary radiation balance. This gas is a direct outcome of air pollution on the surface. The sinking of stratospheric O3 from the upper atmosphere toward the surface of the Earth is the main natural source of surface O3. In contrast, photochemical processes involving carbon monoxide (CO), such as those that occur in smog, are the main human-driven source of surface O3.
The primary photochemical reactions with or involving harmful Carbon Monoxide are the source of this gas. However, unlike the aforementioned well-known greenhouse gases, this gas has no normal option for falling, making it one of the most dangerous gases present near the earth's surface. Ozone is also the most major low-level or surface greenhouse gas.
The best estimates place the natural surface O3 content at 10 ppb, while the net radiative forcing brought on by anthropogenic surface O3 emissions is roughly 0.35 watts per square meter. In cities that are prone to photochemical smog, ozone concentrations can approach harmful levels (conditions when concentrations meet or exceed 70 ppb for eight hours or longer).