Top 4 Best Online Clinical Data Management Courses
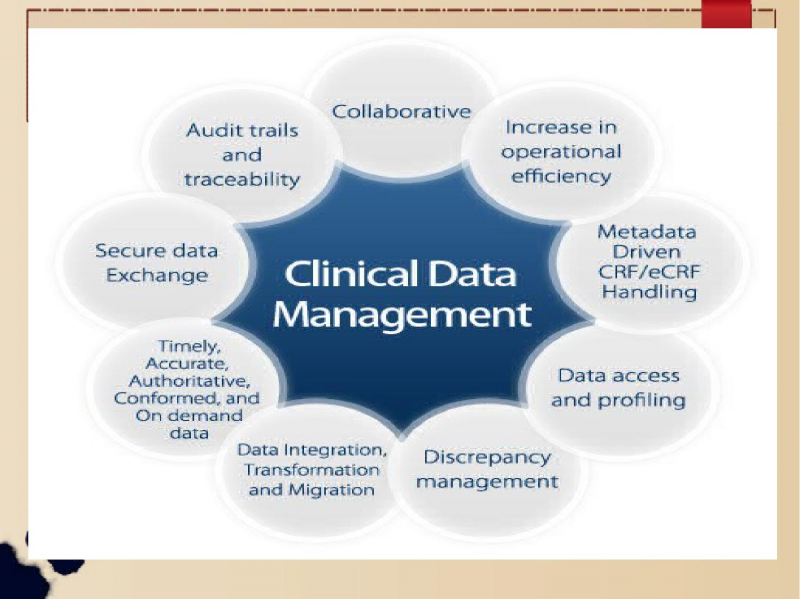
Clinical Data Management (CDM) is an important technique in clinical research that generates statistically sound, reliable, and high-quality data from various ... read more...clinical tests. Data from various clinical trials is collected, analyzed, and integrated at an appropriate quality and cost. Clinical Data Management is the process of entering, verifying, validating, and controlling data collected during clinical trials. Clinical Data Management is an important aspect of any clinical trial. Toplist has compiled a list of the Best Online Clinical Data Management Courses if you're looking for one. Take a look!
-
Basic of Clinical Data Management comes next on the list of the Best Online Clinical Data Management Courses. The course will go over all of the important topics related to Clinical Data Management and its fundamentals in detail. The ten modules would lead systematically to the depth of each specific subject. In the end, you will be brimming with knowledge about Clinical Data Management, which will be extremely useful when you begin working as a clinical research professional in a CRO or pharma company.
What you will learn: After enrolling in the course, you will learn about the basics of clinical data management. This course includes lots of examples of how a clinical data manager works. Courses content: Introduction to CDM; Clinical Trial Phases in Clinical Data Management; Case Report Form; Clinical Data Management System, Data Entry; Data Cleaning; Medical Coding; Data Cleaning External Data; SAE Reconciliation; Database Lock.
Rating: 4.3/5 (206 ratings)721 students
Price: $13.99 (Original price$69.99) Get a 30-day money-back guarantee
Total duration: 1h 23min
Self-paced: Progress at your own speed
Language: English
Video transcript: English
Prerequisites:
- A Life Science professional willing to work in the Clinical Data Management sector
Who is this course for?
- Life Science, Nursing, Pharmacy, or similar graduates
This course includes:
- 5hour video on demand
- Unlimited access
- Mobile and TV access
- End of training certificate
Enroll here: https://www.udemy.com/course/basic-of-clinical-data-management/
issuu.com 
novofrontcareers.com - A Life Science professional willing to work in the Clinical Data Management sector
-
Finally, The Simplest Guide to Clinical Trials Data Analysis with SAS is one of the most must-check Online Clinical Data Management Courses. This course provides an introduction to the Pharmaceutical/Life Science industry in an easy-to-understand visual and simple format. It demonstrates how SAS is used as a tool in this industry to work with vast amounts of clinical data. The course walks you through an example clinical study sample data and generates different Clinical Study Reports that are submitted to the FDA (in the United States) or other regulatory authorities in other countries. You will not only improve your SAS programming skills, but you will also learn important concepts for working in the pharmaceutical industry, such as biostatistics and clinical data management.
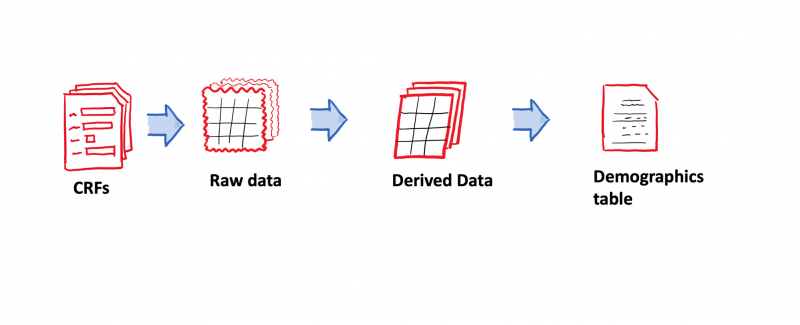
Following an introduction to the pharmaceutical industry and the learning of relevant Clinical Trial concepts, the course takes you through a hands-on training exercise to build the very important and fundamental Clinical Study Report known as the Demographics Table.
You will start with a sample clinical study data in an Excel sheet, import it into SAS, derive all necessary variables as shown in the mock table, and then generate a clinical study report. All of this will be accomplished through the use of guided SAS Programming steps with detailed explanations at each stage of programming. You will have learned to work with Clinical Study Data, generate a real Clinical Study Report, and extend those steps to build other reports that constitute Clinical Trial submissions to regulatory bodies by the end of this course.
Rating: 4.3/5 (598 ratings)2,544 students
Price: $12.99 (Original Price$84.99) Get a 30-day money-back guarantee
Total duration: 2h 3m
Self-paced: Progress at your own speed
Language: English
Video transcript: English
Prerequisites:- Basic SAS Programming
- No prior Pharmaceutical experience is necessary
- Internet connectivity for working in the SAS Studio Edition using SAS OnDemand (no installation necessary)
Who is this course for?
- Anyone wanting to get into the Life Science / Pharmaceutical industry and looking to work in this field
- Want to take up a job as a Clinical SAS Programmer in a Pharma company or a CRO (Contract Research Org.)
- Want to just play with data using the SAS Programming concepts
This course includes:
- 2 hours on-demand video
- 3 downloadable resources
- 1 practice test
- Full lifetime access
- Access on mobile and TV
- Assignments
- Certificate of completion
Enroll here: https://www.udemy.com/course/the-simplest-guide-to-clinical-sas-programming/
skillshare.com -
One of the top-pick Online Clinical Data Management Courses in Udemy is the Clinical Data Management Course created by Anuj Tripathi. It will provide you with the knowledge and skills you need to succeed in your career. Clinical Data Management (CDM) is a crucial stage in Clinical Research that results in the generation of high-quality, reliable, and statistically sound data from clinical trials. Clinical data management facilitates the collection, integration, and availability of data while maintaining appropriate quality and cost. It also helps with the design, management, and analysis of clinical research studies. This program will assist you in comprehending the technical aspects of the Clinical Data Management process.
What you will learn: Work with the world's leading Contract Research Organizations as a Clinical Data Management Professional. Courses content: Introduction, CDM System, Medical Coding in CDM, Clinical Data Process, Case Report Form, Quality & Regulatory System in CDM.
Rating: 3.9/5 (307 ratings)1,160 students
Price: $18.99 (Original price $109.99) Get a 30-day money-back guarantee
Total duration: 2h14 min
Self-paced: Progress at your own speed
Language: English
Video transcript: English
Prerequisites:
- A Life Science professional willing to work in the Clinical Data Management sector
Who is this course for?
- Science, Pharma or Medical Graduate, Clinical Research and IT Professionals
This course includes:
- 2-hour video on demand
- Unlimited access
- Mobile and TV access
- End of training certificate
Enroll here: https://www.udemy.com/course/clinical-data-management-cdm-online-course/

icriindia.com 
aitrends.com -
This course provides a basic understanding of clinical data management and the processes involved in producing high-quality clinical trial data, which aids in product commercialization. This Comprehensive Course Teaches the Fundamentals of Clinical Data Management: Learn about Clinical Data Management and its phases and activities. Identify CDM tools and roles; CDM activities are used by the master to manage clinical data.
Clinical Data Management is critical to the overall research function because the data to support the submission is its primary deliverable. CDM has evolved in response to the growing demand from pharmaceutical companies to accelerate the drug development process and from regulatory authorities to put in place quality systems to ensure the generation of high-quality data for accurate drug evaluation.
This course is intended for beginners in the healthcare industry to introduce the concept of clinical data management and data management activities through 24 video lectures with 2 hours of content and two end-of-module quizzes. Section 1 begins with an overview of clinical data management, discussing what clinical data management is and the regulations and standards that are implemented in CDM. Section 2 describes the CDM process, data management plan, and activities involved in different phases of data management. Section 3 provides an overview of the various tools used in CDM, as well as key members of the CDM team and their responsibilities.
You will be able to: Understand clinical data management and its role in clinical research by the end of the course; Describe the CDM process activities. Demonstrate the functions of key CDM members and the various CDM tools available.
Rating: 3.5/5 (75 ratings)228 students
Price: $13.99 (Original price$69.99) Get a 30-day money-back guarantee
Total duration: 1h 23min
Self-paced: Progress at your own speed
Language: English
Video transcript: English
Prerequisites:- Prior knowledge of clinical terminology is an added advantage but not a requirement
Who is this course for?
- The target audience for this course is healthcare professionals
This course includes:
- 2 hour video on demand
- Unlimited access
- Mobile and TV access
- End of training certificate
Enroll here: https://www.udemy.com/course/basic-of-clinical-data-management/

baltictimes.com 
leverageedu.com