Top 7 Things to Know About Entyvio
Entyvio is used to treat inflammation in ulcerative colitis and Crohn's disease. Entyvio is to be administered intravenously with the prescription and ... read more...supervision of a physician. Dosage and notes when using Entyvio will be in the article below.
-
Entyvio is a brand name (trade name) for vedolizumab, a biologic therapy used to treat ulcerative colitis and Crohn's disease.
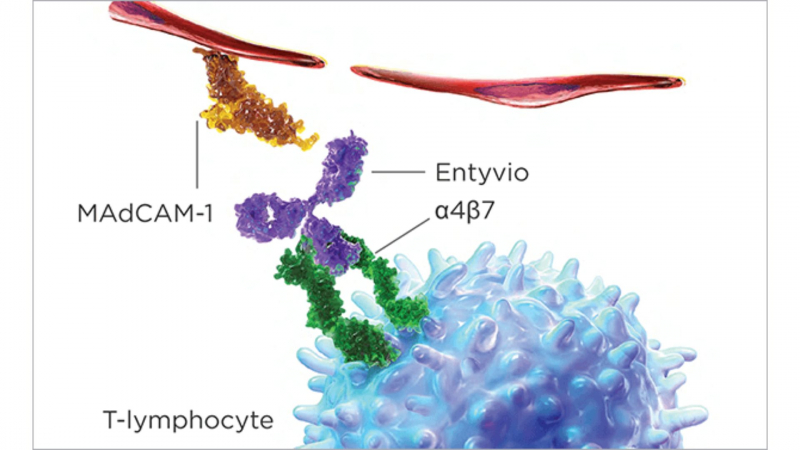
Entyvio (vedolizumab) binds to the alpha4beta7 integrin on the surface of T-lymphocytes and inhibits its interaction with the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) to prevent memory T-lymphocytes (a type of white blood cell) from migrating across the endothelium into inflamed gastrointestinal tract tissue. The interaction of alpha4beta7 integrin with MAdCAM-1 has been identified as a significant contributor to the chronic inflammation seen in ulcerative colitis and Crohn's disease. Simply put, Entyvio reduces the number of white blood cells that enter your GI tract (intestine), which reduces inflammation and the signs and symptoms of Crohn's or ulcerative colitis.
Entyvio is a member of the group of medicines known as integrin receptor antagonists. It can also be referred to as a monoclonal antibody or a selective immunosuppressant.
Entyvio® (vedolizumab) 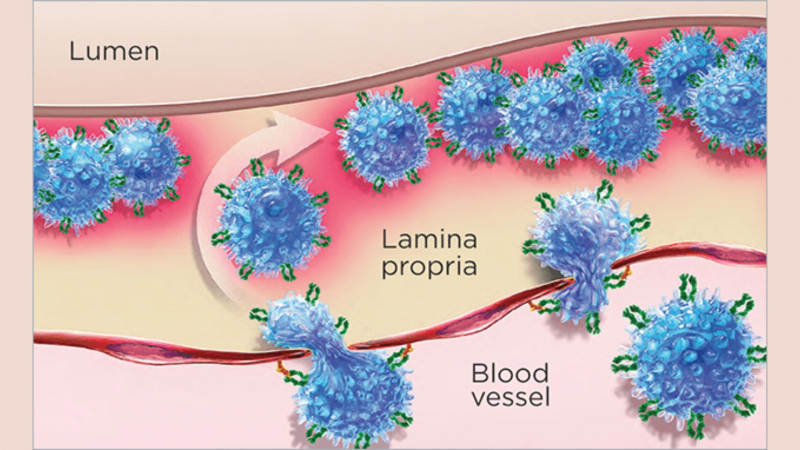
Entyvio® (vedolizumab) -
Adults with moderate to severe ulcerative colitis or Crohn's disease may benefit from this medication.
Entyvio has no known systemic (whole-body) immunosuppressive effects, but it is likely to increase a person's susceptibility to infections.
Unlike some other biologics, it specifically targets the gut rather than the entire body.
Entyvio's maintenance dose is typically 300mg given as an infusion every two months (8 weeks).
When older people over the age of 65 were given Entyvio, there were no differences in side effects or response.
Entyvio® (vedolizumab) 
Ausmed -
If you are between the ages of 18 and 60, do not take any other medications, and have no other medical conditions, you are more likely to experience the following side effects:
- The most common side effects reported with this medication are infections (such as a cold, flu, sinus infections, or bronchitis), headache, pain (in the throat, joints, arms, or legs), nausea, fever, tiredness, cough, rash, and itching.
- Weight gain was not reported as a side effect of Entyvio in the manufacturer's clinical trials. When gastrointestinal diseases such as Crohn's disease or ulcerative colitis improve, patients who had previously lost weight may gain some weight.
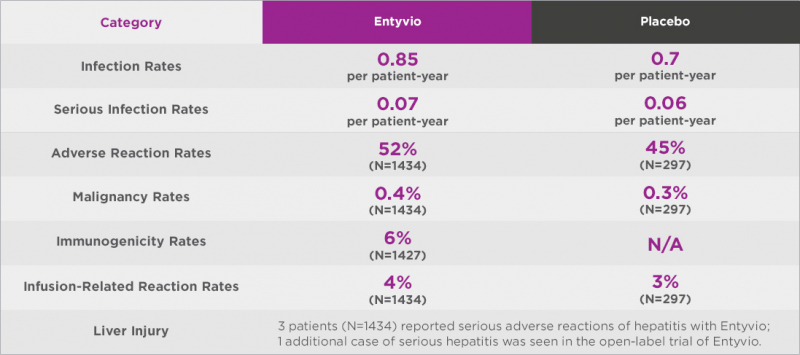
- Entyvio is linked to a higher risk of infections, some of which may be serious, despite the fact that it is not regarded as a whole-body immunosuppressant. Anal abscess, sepsis (some fatal), tuberculosis, salmonella sepsis, listeria meningitis, giardiasis, and cytomegaloviral colitis were among the serious infections reported in patients treated with Entyvio. The common cold (13%) and upper respiratory tract infections (7%), respectively, were the infections that were most frequently reported in clinical trials. Other infections included sinusitis (3%), bronchitis (4%), and influenza (4%). Tysabri (natalizumab) and corticosteroids are additional medications that raise the risk of contracting Entyvio infection (eg, prednisone or methylprednisolone).
- Entyvio carries a risk of progressive multifocal leukoencephalopathy (PML). PML is a fatal or severely disabled opportunistic viral infection of the brain caused by the JC virus (JCV).
- Serious allergic reactions can occur during an Entyvio infusion or for several hours after treatment. Side effects should be monitored in all patients during and shortly after the Entyvio infusion.
- Some people may develop liver problems as a result of Entyvio. Liver problems may cause a loss of appetite, which may result in weight loss.
- Entyvio is expensive, with a supply of one 300 mg vial of powder for injection costing around $6,700. Entyvio may be available for as little as $5 per dose for those with private commercial insurance. If you do not have insurance, Takeda may be able to help you financially. Patients with government-sponsored insurance, such as Medicare, Medicaid, or TriCare, are not eligible for the $5 copay program, but they may be able to get help from an independent copay foundation.
- A healthcare provider must administer it as an intravenous infusion over approximately 30 minutes. It cannot be administered as a push, IM, or SC injection.
- A small number of people who receive Entyvio may develop antibodies to it. In the UC and CD trials, 4% (56) of patients developed antibodies to Enyvio, with 9 remaining positive. Entyvio concentrations were undetectable in six of the nine patients, with two having lower concentrations. In the controlled trials, none of these nine patients achieved clinical remission at Weeks 6 or 52.
- A person's vaccination status should be up to date before receiving Entyvio.
- If a person is not benefiting from Entyvio after 14 weeks, the medication should be stopped.
- Entyvio use in pregnant women has not been linked to an increased risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Uncontrolled inflammatory bowel disease poses a risk to both the mother and the fetus. Calculate the risks versus the benefits. Any woman who becomes pregnant should call 1-877-TAKEDA7 (1-877-825-3327) to join the pregnancy registry. According to the data, Entyvio is detectable in breast milk. The consequences of this exposure on a nursing infant are unknown. Consider the advantages and disadvantages.
- There is currently no generic Entyvio available.
Seniors and children, people with certain medical conditions (such as liver or kidney problems, heart disease, diabetes, seizures), or people who take other medications are more likely to experience a broader range of side effects.

Ausmed 
Dr Ram Chandra Soni -
Entyvio is an integrin receptor antagonist that may be used to treat moderate to severe ulcerative colitis or Crohn's disease. It specifically targets the gut rather than the entire body, which may reduce its systemic (whole-body) immunosuppressive effects, but it is still likely to increase an individual's risk of infection.
Medpage Today 
www.entyvio.com -
Entyvio is infused into your vein as a 300 mg intravenous (IV) infusion. Your infusions will be administered at your doctor's office, a local clinic, a hospital, or even at your home. Consult your doctor and insurance company to determine which location is the best and most affordable for you. Entyvio infusions take about 30 minutes each, but your entire clinic visit could last several hours. After the infusion, you should be able to resume your normal activities.
Tell your doctor if you suspect an infection or exhibit symptoms of an infection, such as fever, chills, muscle aches, cough, shortness of breath, runny nose, sore throat, red or painful skin or sores on your body, fatigue, or pain when urinating, both before beginning treatment with Entyvio and while you are receiving it. Let them know if you have recurring infections as well.
You will receive three 30-minute starting dose infusions. To begin treatment, you will receive these infusions at week 0 (your first dose), week 2, and week 6. The majority of patients are given 300 mg per dose. Following your initial doses, you will receive one 30-minute infusion every 8 weeks to keep your disease under control.
There have been reports of severe hypersensitivity reactions to Enytvio. A rash, itching, swelling of your lips, tongue, throat, or face, shortness of breath or difficulty breathing, wheezing, dizziness, feeling hot, or palpitations are all symptoms of an allergic reaction (racing heartbeat). If you have these symptoms after leaving the clinic, call 911 or have someone take you to the nearest emergency room.
Inform your doctor. If you have a history of severe infections. Before beginning Entyvio treatment, your doctor may also screen you for tuberculosis (TB). You may also need to have your vaccines updated. Unless your doctor approves, you should avoid live vaccines once you begin Entyvio treatment.
Consult your doctor if you suspect an infection or have symptoms such as fever, chills, muscle aches, cough, shortness of breath, runny nose, sore throat, red or painful skin, sores on your body, tiredness, or pain while urinating.
Some people may develop liver problems as a result of Entyvio. Notify your doctor right away if you have any of the following symptoms: tiredness, loss of appetite, pain on the right side of your stomach (abdomen), dark urine, or yellowing of the skin or eyes (jaundice).
Inform your doctor about all of the medications you use, including prescription and over-the-counter medications, vitamins, and herbal supplements.
There is no evidence to suggest that taking Entyvio while pregnant increases the risk of serious birth defects, miscarriage, or other risks to the unborn child. Nevertheless, it is generally advised against using medications while pregnant. If you unintentionally fall pregnant while taking Enytvio, tell your doctor right away, and sign up for the pregnancy registry (call 1-877-TAKEDA7 [1-877-825-3327]). Additionally, evidence suggests that Entyvio can be found in breast milk. Unknown are the effects of this exposure on a nursing infant.

Verywell Health 
Moustarah & Company -
Entyvio may lessen some ulcerative colitis (UC) symptoms within a few weeks, but it could take up to six weeks to start noticing a difference. Entyvio can significantly reduce symptoms, bring about remission, and reduce or eliminate the need for corticosteroids with continued use. Symptoms like cramping and pain in the stomach, diarrhea, and fatigue disappear when a person is in remission.
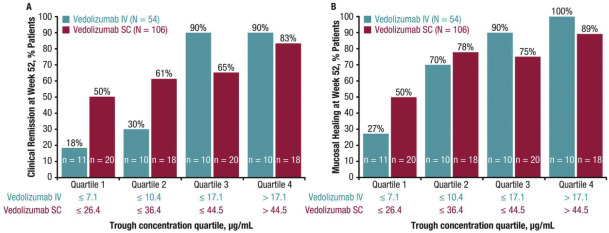
In one trial, 46% (225 patients) of 373 patients with UC who received Entyvio had a clinical response at 6 weeks, compared to 26% (149 patients) who received a placebo (inactive agent). At 6 weeks, some patients achieved clinical remission (17% on Entyvio vs. 5% on placebo). In one-year studies, 42% of patients were in clinical remission at Week 52, compared to 16% of patients on a placebo.
In two Entyvio studies that were extended to a year, 39% and 44% of patients were in remission at the end of the trial, compared to 22% and 30% of placebo patients. Both studies yielded statistically significant results.
In 2 studies, 15% of Crohn's disease patients receiving Entyvio were in remission at 6 weeks, compared to 7% and 12% of patients receiving placebo. It was determined that only one study was statistically significant.
Each patient has a unique response to medication therapy and a range of disease severity. Entyvio won't work on all patients.
Your doctor may think about stopping your Entyvio treatment if, after 14 weeks, you have not noticed a therapeutic benefit from it.
ScienceDirect.com 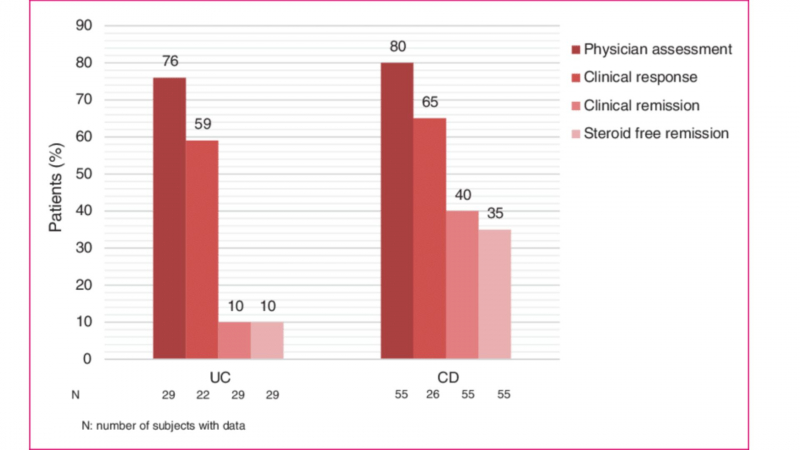
Efficacy of vedolizumab induction therapy in ulcerative colitis (UC) (week 10) and Crohn's disease (CD) (week 14). - ResearchGate -
Medicines that interact with Entyvio may reduce its effect, shorten its duration of action, increase side effects, or have no effect when combined. An interaction between two medications does not always necessitate the discontinuation of one of them; however, it can. Consult your doctor about how to handle drug interactions.
Entyvio may interact with the following medications:
- TNF blockers, such as adalimumab, etanercept, golimumab, or infliximab
- corticosteroids (such as prednisone or dexamethasone)
- herbals, such as black cohosh
- immunosuppressants such as azathioprine, cyclosporine, or tacrolimus
- interferon
- live vaccines and some other vaccines, such as BCG, cholera, measles, hepatitis b vaccines, yellow fever, or live influenza vaccines (may be administered concurrently if the benefits outweigh the risks)
- multiple sclerosis medications, such as fingolimod
- probiotics, such as lactobacillus
- tumor necrosis factor (TNF) blockers, such as natalizumab.
Please keep in mind that this list is not exhaustive and only includes common medications that may interact with Entyvio. For a complete list of Entyvio interactions, consult the prescribing information.
YouMed 
Vinmec