Top 7 Things to Know About Farxiga
Farxiga is a medication used in type 2 diabetes to control blood sugar. In addition, Farxiga is also used to reduce the risk of death from heart failure and ... read more...stroke in people with type 2 diabetes and heart disease. Dosage and ventilation when using Farxiga will be in the shared article below.
-
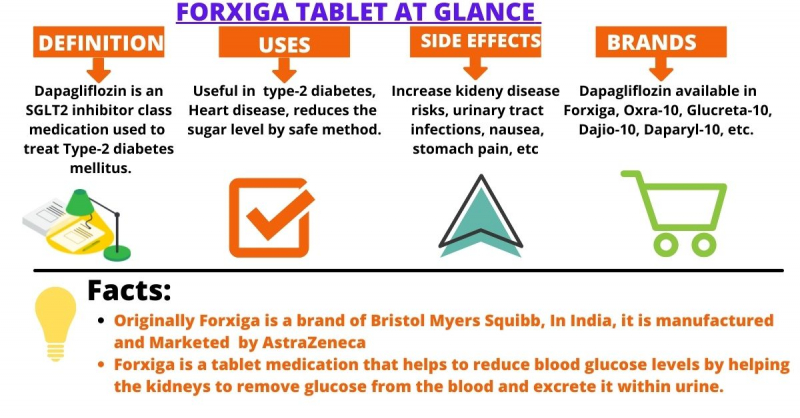
Farxiga is the brand name (trade name) for dapagliflozin, which may be used to treat type 2 diabetes.
Dapagliflozin works by inhibiting the sodium-glucose co-transporter 2 (SGLT2), a protein found in the early proximal tubule of a nephron (a kidney cell) that is responsible for the reabsorption of glucose back into circulation from the blood that flows through the kidneys (the glomerular filtrate). Dapagliflozin prevents glucose resorption and increases the amount of glucose excreted in the urine by blocking this transporter. This aids in the reduction of blood sugar and the improvement of A1C. Dapagliflozin also reduces sodium reabsorption and increases sodium delivery to the distal tubule, which can help reduce heart loading and how hard the heart has to work to pump blood around the body.
Farxiga belongs to a class of drugs known as SGLT-2 inhibitors.
Breathe Well-Being 
American College of Cardiology -
Along with diet modification and increased exercise, it may be used to treat type 2 diabetes.
It can also be used to lower the risk of heart failure hospitalization in people with type 2 diabetes who also have cardiovascular disease or risk factors for cardiovascular disease.
Farxiga is also approved to treat chronic kidney disease (CKD) in people with or without type 2 diabetes who are at risk of progression. This approval represents the most significant advance in the treatment of chronic kidney disease in over 20 years.
It is effective at lowering blood glucose levels, HbA1c, and body weight.
May be used in conjunction with other diabetes medications such as insulin, metformin, or sulfonylureas; however, the dosage of these medications may need to be reduced due to an increased risk of hypoglycemia with combination treatment.
In patients with an eGFR greater than 45 mL/min/1.73m2, no dosage adjustment is required.In people with liver disease, no dosage adjustments are required; however, Farxiga has not been extensively studied in patients with severe liver disease.
Most Farxiga users lose between 2.6 and 2.7 kg of weight. Some people, however, may gain weight.
Farxiga is administered orally.

Canadian Pharmacy Online 
Medical Dialogues -
If you are between the ages of 18 and 60, do not take any other medications, and have no other medical conditions, you are more likely to experience the following side effects:
- Low blood sugar levels, low blood pressure (especially when starting Farxiga), genital and urinary tract infections, a congested nose, back pain, increased urination, nausea, and high cholesterol are all common Farxiga side effects.
- Patients with mild-to-moderate kidney disease have an increased risk of bone fracture.
- Farxiga is not recommended for people with type 1 diabetes or for the treatment of diabetic ketoacidosis.
- Farxiga increases urination, which can result in dehydration or a drop in blood pressure. Before starting Farxiga, any dehydration or volume depletion should be corrected. Those with volume depletion, seniors with kidney disease, or those who use diuretics are more vulnerable.
- Individuals with an eGFR less than 30 mL/min/1.73m2 who are being treated for glycemic control without established CV disease or multiple CV risk factors are contraindicated. Low blood pressure, bone fractures, and acute kidney injury are more common in people with an eGFR of 30-60 mL/min/1.73m2. Dehydration, taking certain medications, such as NSAIDs, ACE inhibitors, ARBs, or diuretics, or having a low-calorie intake may increase the risk. Recent research has shown that Farxiga when given to people with CKD, can reduce the risk of renal function worsening, end-stage kidney disease, and cardiovascular or renal death.
- Because it is not expected to be effective in these populations, it is not recommended for the treatment of CKD caused by polycystic kidney disease or in people who have previously received immunosuppressive therapy for kidney disease.
- Farxiga has been linked to an increased risk of diabetic ketoacidosis (a severe lack of insulin), which can be fatal.
- This could be due to severe metabolic acidosis. If this occurs, treatment for ketoacidosis should be initiated immediately, and Farxiga should be discontinued. Those with pancreatic insulin deficiency, on a calorie-restricted diet, or who consume an excessive amount of alcohol are more vulnerable. It should be noted that the presenting blood glucose levels were lower than those seen with diabetic ketoacidosis (often less than 250 mg/dL).
- Farxiga treatment increases the risk of urinary tract infections, genital fungal infections, and a rare necrotizing infection called necrotizing fasciitis of the perineum (Fournier's gangrene), which causes genital pain, tenderness, redness, and swelling.
- Farxiga treatment may cause an increase in LDL cholesterol levels.
- Farxiga is not recommended during the second and third trimesters of pregnancy, and there is insufficient data to assess the risk during the first trimester. The risk of Farxiga use in the first trimester, however, should be balanced against the risk of uncontrolled diabetes in the fetus in terms of birth defects and miscarriage.
Seniors and children, people with certain medical conditions (such as liver or kidney problems, heart disease, diabetes, seizures), or people who take other medications are more likely to experience a broader range of side effects.

Everyday Health Pharmacist Conversations -
Farxiga is an oral medication used to lower blood glucose levels in people with type 2 diabetes, often in conjunction with other medications. It may also be given to people with chronic kidney disease to reduce the risk of worsening renal function, end-stage kidney disease, and cardiovascular or renal death (CVD). An increased risk of genital and urinary tract infections is one of the side effects.

Verywell Health 
Medicina y Salud Pública -
Farxiga is typically taken in the morning, with or without food. If you need more blood sugar control, are tolerating Farxiga well, and do not have kidney disease, your doctor will start you on 5mg and may increase the dose to 10mg.
If you have a history of heart disease, poor circulation or nerve damage, liver problems, urinary problems, are planning surgery, or are on a low sodium diet, tell your doctor before starting Farxiga.
Farxiga should be taken exactly as directed. If you forget to take a dose, take it as soon as you remember, but not if it is less than eight hours before your next dose; otherwise, resume your regular dosing schedule.
If your doctor has prescribed any other medications, you should also take farxiga. Additionally, you should follow any dietary advice and engage in regular physical activity. If you experience any type of infection, have surgery planned, or are under a lot of stress, consult your doctor right away because your medication needs may change.
Your blood pressure could drop if you take farxiga. This is more likely to happen when you first start taking Farxiga, in seniors, in individuals who already have low blood pressure, in individuals who follow a low sodium diet, and in individuals who take diuretics. You might become lightheaded or dizzy as a result. If this occurs, consult your doctor.
If you experience any of the warning signs or symptoms of diabetic ketoacidosis, such as excessive thirst, frequent urination, abdominal pain, fruity breath, or severe metabolic acidosis, such as nausea and vomiting, shortness of breath, or fatigue, call your doctor right away. Inform your loved ones of the warning signs of metabolic acidosis and diabetic ketoacidosis.
A higher risk of urinary tract infections has been linked to farxiga. Consult your doctor if you experience symptoms like back or pelvic pain, fever, burning or painful urination, increased frequency of urination, or increased frequency of urination.
Farxiga may also increase your chances of developing genital infections. A white or yellowish vaginal discharge, itching, or odor are all symptoms of a vaginal yeast infection. A yeast infection of the penis can cause redness, itching, swelling, a foul-smelling discharge, and penile pain.
If you plan to become pregnant while taking Farxiga, consult with your doctor first.
Diabetes Self-Management 
Global Pharmacy Plus -
Farxiga causes an increase in glucose excretion in the urine very soon after the first dose. After 12 weeks of treatment, dapagliflozin doses of 5mg or 10mg per day resulted in an average of 70 grams of glucose per day excreted in the urine in patients with type 2 diabetes.
Farxiga reaches its peak concentration within two hours of administration. Although food can reduce Farxiga's peak concentration and lengthen the time it takes to reach maximum concentrations, these changes are not clinically significant, and Farxiga can be administered with or without food.
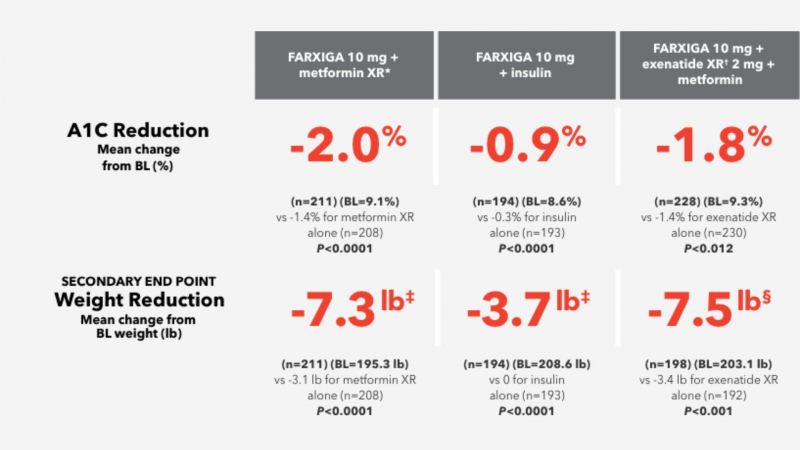
The average reduction in HbA1C from baseline for Farxiga 5mg and Farxiga 10mg after 24 weeks of treatment was 0.8 and 0.9, respectively. For Farxiga 5mg and 10mg, the reduction in fasting plasma glucose from the starting point was 24.1 and 28.8, respectively. Usually, a combination therapy increased response.
Farxiga significantly decreased the risk of the composite of worsening renal function, end-stage kidney disease, and cardiovascular or renal death by about 39% in the DAPA-CKD Phase III trial, which was carried out in patients with chronic kidney disease.
FARXIGA 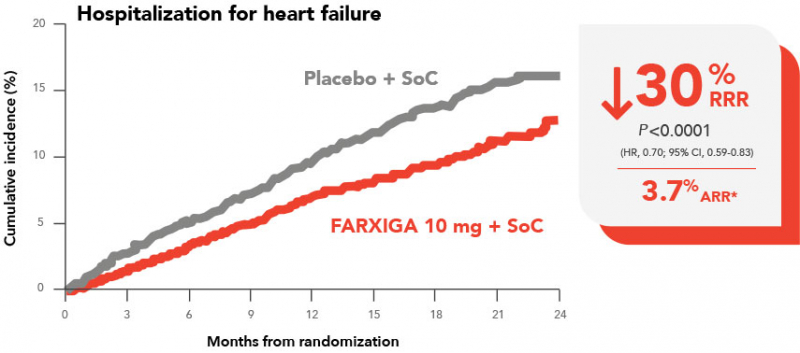
Efficacy in HFrEF Patients - FARXIGA -
When combined with Farxiga, drugs that interact with it either lessen its effect, shorten its duration of action, exacerbate side effects, or have no effect at all. Even though it is not always necessary to stop taking one of the drugs, sometimes there is an interaction between two drugs. Consult your doctor to learn how to handle drug interactions.
The following are typical drugs that may interact with Farxiga:
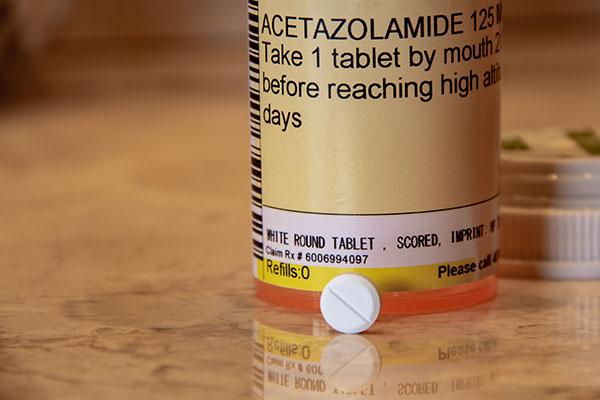
- acetazolamide
- anticonvulsants such as phenytoin or phenobarbital
- antipsychotics, such as aripiprazole or clozapine
- ARBs such as valsartan
- beta-blockers, such as atenolol, labetalol, and metoprolol, may enhance the hypoglycemic effects
- ciprofloxacin or gatifloxacin
- corticosteroids, such as prednisone or cortisone
- digoxin
- diuretics, such as bumetanide, HCTZ, and bendroflumethiazide, which may enhance the potential for volume depletion
- HIV medications, such as amprenavir, atazanavir, fosamprenavir, and ritonavir
- hormones, such as ethinylestradiol and hydroxyprogesterone
- insulin (may increase risk of hypoglycemia)
- isoniazid
- NSAIDs, such as mefenamic acid
- rifampin
- other medications that affect blood sugar levels or are used for diabetes, such as glimepiride, or metformin.
Urine glucose tests should not be used to monitor glucose control in people taking SGLT2 inhibitors like Farxiga because they increase urinary glucose excretion and cause positive urine glucose tests. Alternative glucose control methods should be used. Interference with 1,5-AG assays is also possible.
Please keep in mind that this list is not exhaustive and only includes common medications that may interact with Farxiga. For a complete list of interactions, consult the Farxiga prescribing information.
usada 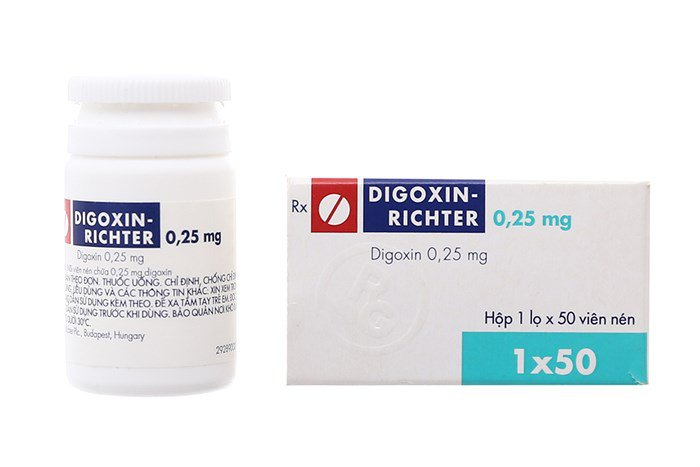
Vinmec