Top 7 Things to Know About Gilenya
Gilenya is a type of medicine known as a ‘disease-modifying therapy’ that is used to treat adults and children over 10 years of age with highly active ... read more...relapsing-remitting multiple sclerosis (MS), a disease of the nerves in which inflammation destroys the protective sheath surrounding the nerve cells. ‘Relapsing-remitting’ means that the patient has flare-ups of symptoms (relapses) followed by periods of recovery (remissions). Gilenya is used when the disease remains active despite appropriate treatment with at least one other disease-modifying therapy, or is severe and getting worse rapidly. Gilenya contains the active substance fingolimod. Read the following article to understand more about this drug.
-
Gilenya is a brand name (trade name) for the drug fingolimod, which is used to treat multiple sclerosis.
Gilenya (fingolimod) is thought to work by preventing lymphocytes (a type of immune cell) from migrating from lymph nodes into the bloodstream. Gilenya (fingolimod) is derived from myriocin (ISP-1), a fungus metabolite and natural immunosuppressant. Yoshitomi Pharmaceuticals developed it from ISP-I through chemical modification in 1992.
Gilenya is a type of medicine known as a selective immunosuppressant.
Drugs.com 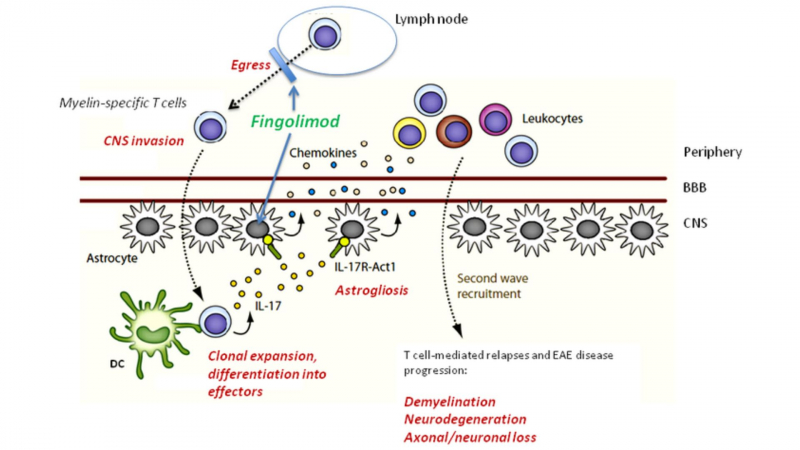
Discovery Medicine -
Gilenya is an immunosuppressant that can be used to treat relapsing multiple sclerosis (MS) in adults and children aged 10 and up.
Gilenya reduces nerve inflammation and subsequent damage.
Gilenya is given at the same dose to adults and children over the age of 10 who weigh more than 40kg: 0.5 mg once daily. For those weighing less than 40kg, the dose should be reduced to 0.25mg once daily.
Gilenya is taken once daily by mouth (orally).
Can be taken with or without food.
For people with mild-to-moderate liver disease, no dosage adjustments are required.

Multiple Sclerosis News Today 
HealthResearchFunding.org -
If you are between the ages of 18 and 60, do not take any other medications, and have no other medical conditions, you are more likely to experience the following side effects:
- Abdominal pain, a rise in blood triglycerides, an increased risk of basal cell carcinoma (2%), skin papilloma (3%), an increased risk of infections like the flu, sinusitis, bronchitis, herpes zoster, and tinea versicolor, back pain or other types of pain, blood disorders, blurred vision, bradycardia (slow heartbeat), cough, diarrhea, hair loss, headache, high blood pressure, liver enzyme
- There have been instances of progressive multifocal leukoencephalopathy (PML) in MS patients who took Gilenya. The JC virus (JCV) causes PML, an opportunistic viral infection of the brain that frequently results in death or severe disability. There have also been reports of respiratory effects and posterior reversible encephalopathy syndrome (PRES).
- Gilenya rarely causes a side effect called hair loss. In clinical trials, Gilenya was taken by 3% of those who experienced hair loss, compared to 2% of those who received a placebo.
- Gilenya should not be administered to patients with a baseline QTc interval of less than 500 msec, with cardiac arrhythmias requiring anti-arrhythmic treatment with Class Ia or Class III anti-arrhythmic drugs, with a history of an allergic reaction to fingolimod or any of the excipients in Gilenya, or with other cardiovascular conditions such as unstable angina, stroke, or severe heart failure.
- Before beginning Gilenya treatment, get a CBC. Through redistribution in secondary lymphoid organs, gilenya reduces peripheral lymphocyte count to 20%–30% of baseline values in a dose-dependent manner. This means that it is impossible to assess the lymphocyte levels in a patient receiving Gilenya therapy using peripheral blood lymphocyte counts. Obtain serum transaminases (ALT and AST) and total bilirubin levels six months after starting Gilenya.
- Before starting Gilenya, patients with pre-existing conditions such as ischemic heart disease, myocardial infarction history, or cerebrovascular disease should undergo a comprehensive cardiac evaluation. Before beginning treatment with Gilenya, conduct a thorough medication review to look for additional drugs that may also slow the heart rate and consider modifying the regimen.
- Gilenya does not cure MS; it only reduces the number of relapses.
- Gilenya suppresses the immune system and reduces an individual's ability to fight infection. This ability will be harmed further if the person is also taking other immune-suppressing drugs, such as chemotherapy, immune-modulating therapies, or other immunosuppressants. This immune-suppressive effect will last for up to two months after you stop taking Gilenya. Before starting Gilenya, review all other immune-suppressing medications, especially those with a long half-life whose effects may last for weeks after discontinuation, such as natalizumab, teriflunomide, or mitoxantrone. Gilenya has been linked to life-threatening and fatal infections.
- Gilenya will also suppress the immune response to live vaccines like MMR and varicella. Before Gilenya is administered, all vaccines should be up to date. Before beginning Gilenya treatment, test patients for antibodies to varicella zoster virus (VZV) and vaccinate all antibody-negative patients with VZV vaccination. If possible, all pediatric patients should complete all immunizations in accordance with current immunization guidelines before beginning Gilenya therapy.
- Gilenya has been linked to an increased risk of macular edema. Perform an eye examination before beginning treatment, again 3 to 4 months later, and at any time a patient reports visual disturbances while on Gilenya therapy.
- Gilenya can cause bradycardia, especially during the first dose. The maximum drop in heart rate usually occurs within 6 hours of the first dose and returns to baseline levels 8 to 10 hours later. Because of physiological diurnal variation, there is also a second period of heart rate decrease within 24 hours. In clinical trials, 0.6% of patients receiving Gilenya 0.5 mg and 0.1% of patients receiving placebo experienced symptomatic bradycardia after the first dose. The majority of people were asymptomatic, but some had hypotension, dizziness, fatigue, palpitations, and/or chest pain.
- Gilenya should be started in a setting where people can be appropriately managed for symptomatic bradycardia (slow heartbeat) for 6 hours (sometimes longer). All patients should have an ECG before the first dose and at the end of the observation period. Every hour for the next 6 hours, the patient's pulse and blood pressure should be taken.
- If any of the following are present, continue monitoring after 6 hours: a heart rate of less than 45 beats per minute in adults, less than 55 beats per minute in pediatric patients 12 years of age and older, or less than 60 beats per minute in pediatric patients 10 or 11 years of age; the heart rate taken 6 hours postdose is at its lowest value, implying that the maximum pharmacodynamic effect on the heart has not occurred; the ECG obtained 6 hours postdose shows new onset second degree or higher AV block. If no pharmacological treatment is required, continue monitoring until symptoms have resolved; otherwise, continue monitoring overnight and repeat 6-hour monitoring after the second dose. Overnight ECG monitoring may be required for some patients.
- All patients must have first-dose bradycardia monitoring if Gilenya is stopped with a one-day or more interruption during the first two weeks or during weeks 3 and 4 of treatment, after treatment interruptions of more than 7 days. For interruptions of more than 14 days, first-dose monitoring is required.
- Gilenya is costly. A supply of 30 capsules of Gilenya 0.5mg costs $9053 or about $302 per capsule. But 96 to 99% of those who have private or public health insurance, are qualified for Medicare, or are on Medicaid are covered for Gilenya. The GILENYA Medical Co-Pay Support Program also offers co-pay assistance. Most people don't have to.
- Pre-test and initial observation expenses might also be covered.
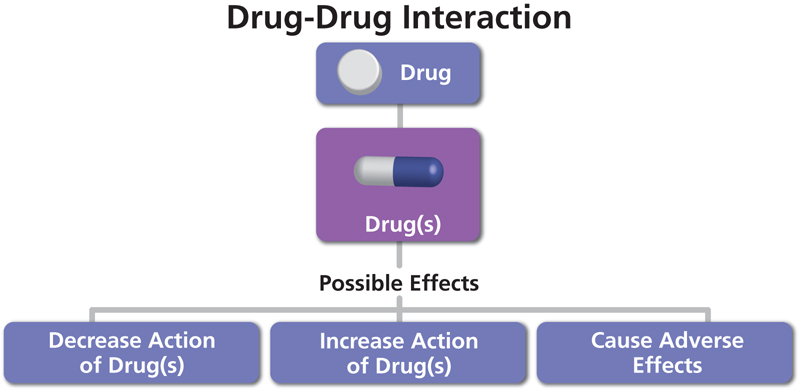
- Numerous medications, particularly those that slow the heart rate or have an impact on the immune system, interact with Gilenya.
- Some Gilenya metabolites may have blood levels that are up to 13 times higher in people with kidney disease. The results of this cannot yet be determined due to a lack of research.
- Gilenya can harm a developing baby, and animal studies have shown developmental toxicity at doses lower than the recommended human dose. To avoid pregnancy during and for two months after stopping Gilenya treatment, all females of reproductive potential should use effective contraception.
Seniors and children, people with certain medical conditions (such as liver or kidney problems, heart disease, diabetes, seizures), or people who take other medications are more likely to experience a broader range of side effects.

Multiple Sclerosis News Today 
Star Health Insurance -
Gilenya is an immunosuppressant that can be used to treat relapsing forms of multiple sclerosis in adults and children over the age of ten. It may increase your risk of infection and interact with other medications, and stopping Gilenya may worsen your MS symptoms. Some of the most common side effects reported include a headache, nausea, diarrhea, and abdominal pain.

Ochsner Blog - Ochsner Health 
CBC -
Gilenya can be taken with or without food, but it is best to take it at the same time every day. If you forget to take a dose, take it as soon as you remember. If your next dose is approaching, skip it; do not double your dose. If you miss a dose during the first two weeks of taking Gilenya, contact your doctor because your heart rate may need to be monitored again.
Gilenya can cause your heart rate to slow, and this is more likely to occur with the first dose of Gilenya or if you miss a dose during the first two weeks of treatment and then restart Gilenya.
Because Gilenya suppresses your immune system, you are more susceptible to infections while taking it. While receiving treatment with Gilenya and for two months after stopping it, some vaccinations may need to be avoided. Be cautious to stay away from sick people and take precautions to keep yourself safe. If you experience any infection-related symptoms, such as fever, pain, swelling, redness, or a discharge, see your doctor right away.
Although Gilenya is expensive, most people pay nothing because it is covered by 96 to 99% of people with commercial or private health insurance or who are eligible for Medicare or Medicaid. The GILENYA Medical Co-Pay Support Program also provides co-pay assistance. Pre-testing and initial observations may also be covered. The Novartis Patient Assistance Foundation, Inc. (NPAF) is dedicated to providing access to Novartis medications to those in financial need or without prescription coverage. Call 1 800-445-3692 to reach the Gilenya Go Program.
Do not stop taking Gilenya without first consulting your doctor. In 2018, the FDA issued a warning stating that stopping Gilenya could cause some people's MS to worsen significantly. This deterioration is uncommon, but it has the potential to result in permanent disability. The increase in disability generally occurred within 12 weeks of stopping Gilenya, but it was reported up to 24 weeks later. Stopping may be due to intolerable adverse drug reactions, a planned or unplanned pregnancy, or because the medicine is no longer effective.
Gilenya can increase your risk of macular edema, so you should have an eye exam before starting treatment, 3 to 4 months after starting treatment, and then on a regular basis after that.
If you experience any unusual or concerning side effects, including stomach pain, dizziness, heart palpitations, a rash, nausea, vomiting, shortness of breath, fever, or vision issues, consult your doctor right away. A serious viral infection called progressive multifocal leukoencephalopathy (PML), whose symptoms include progressive weakness on one side of the body, clumsiness in the limbs, vision problems, changes in thinking, memory, and orientation that cause confusion, and personality changes, can also increase your risk of developing while taking Gilenya. If you experience any of these symptoms, consult a doctor right away.
Gilenya is not safe to use during pregnancy and should not be used while breastfeeding. If you are a woman of childbearing age, you should get a pregnancy test before starting Gilenya and use reliable contraception while taking it. If you become pregnant while taking Gilenya or within two months of stopping it, notify your doctor immediately.
Gilenya can increase your chances of getting skin cancer, such as basal cell carcinomas and melanomas. Wear protective clothing and use sunscreen with a high sun protection factor to protect your skin from the sun. Inform your doctor if you notice any changes in the appearance of your skin, such as changes in a mole or persistent sores.
Gilenya capsules should be stored at room temperature 20ºC to 25ºC (68ºF-77ºF) and kept dry.
Verywell Health 
InsuranceDekho -
In a study of over 1200 people with MS, the annualized relapse rate was significantly lower in patients treated with Gilenya than in patients who received placebo (an inactive treatment). After two years, 70% of those taking the drug reported no relapse, compared to 46% of those taking a placebo. Over a 24-month period, the mean (median) number of new or newly enlarging T2 lesions was 2.5 with Gilenya and 9.8 with placebo. Other studies have yielded comparable results.
Gilenya stays in the blood for a long time, and it takes 1 to 2 months for lymphocyte counts to return to normal. A person's immune system will remain compromised for up to two months after stopping Gilenya treatment, increasing their risk of infection.
Gilenya reduces blood lymphocytes to approximately 60% of baseline within four to six hours of the first dose, according to research. With continued dosing, the lymphocyte count decreased until it reached a low point (known as the nadir count) of blood lymphocyte count that corresponded to 30% of baseline or 500 cells/mcL after two weeks.
On at least one occasion, some studies have reported nadir counts of 200 cells/mL in patients.
Gilenya is a disease-modifying drug that cuts the number of relapses of multiple sclerosis (MS) in half (50%). Any relapses that occur should be milder. Gilenya takes about two weeks to reach its maximum effect, which is a lymphocyte count of 30% of baseline, or around 500 cells/mcL.
There is a chance that if you stop taking Gilenya, your MS symptoms will worsen. This effect is extremely rare, with only 35 documented cases in the last 8 years. Recovery times varied. 17 patients recovered partially, 8 experienced permanent disability or no recovery, and 6 eventually returned to the level of disability they had before or during Gilenya treatment. Several patients who could walk without assistance before stopping Gilenya ended up needing wheelchairs or becoming completely bedridden.
Gilenya doses greater than 0.5mg once daily are associated with more side effects and little additional benefit.

Multiple Sclerosis News Today 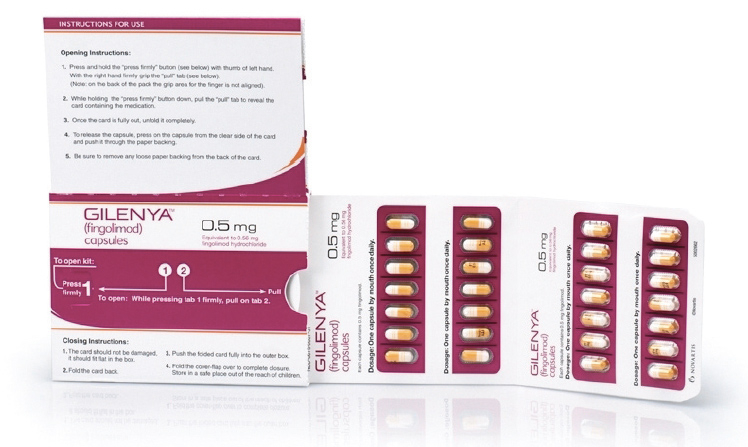
Pharmaphorum -
Medicines that interact with Gilenya may reduce its effect, shorten its duration of action, increase side effects, or have no effect when combined. An interaction between two medications does not always necessitate the discontinuation of one of them; however, it can. Consult your doctor about how to handle drug interactions.
Gilenya interacts with over 500 medications, the vast majority of which are considered major. Among the medications that may interact with Gilenya are:
- antibiotics such as azithromycin, ciprofloxacin, or norfloxacin
- antineoplastics, such as capecitabine, or cyclophosphamide
- antipsychotics, such as clozapine, aripiprazole, or haloperidol
- astemizole
- biologics, such as adalimumab, etanercept, golimumab, or infliximab
- busulfan
- corticosteroids (such as prednisone or dexamethasone)
- heart medications, particularly those that also slow the heart rate, such as beta-blockers (eg, atenolol, sotalol), digoxin, amiodarone, or flecainide (may be associated with severe bradycardia or heart block)
- herbals, such as black cohosh or brewer's yeast
- HIV medications, such as atazanavir or zidovudine
- hydroxychloroquine
- immunosuppressants such as azathioprine, cyclosporine, or tacrolimus
- interferon
- ketoconazole (may increase blood levels of Gilenya up to 1.7 fold. Monitor)
- lithium
- live vaccines and some other vaccines, such as BCG, cholera, measles, hepatitis b vaccines, yellow fever, or live influenza vaccines (Gilenya reduces the immune response to vaccination if vaccines are given within 2 months of a Gilenya dose)
- methotrexate
- oxytocin
- probiotics, such as lactobacillus
- promethazine
- quinine
- tamoxifen.
When combined with other medications like anticancer drugs, immune-modulating agents, or immunosuppressive therapies, Gilenya may have additive immune-suppressing effects that could raise an individual's risk for infection. Consider this when switching from medications that have lingering effects on the immune system, such as mitoxantrone, teriflunomide, or natalizumab.
Strong enzyme inducers like carbamazepine, rifampicin, phenytoin, phenobarbital, and St. John's wort have been shown to reduce Gilenya blood concentrations by 40%. This decrease's clinical significance is unknown.
Please keep in mind that this list is not exhaustive and only includes common medications that may interact with Gilenya. For a complete list of interactions with Gilenya, consult the prescribing information.
BioPharma Services 
Healthday