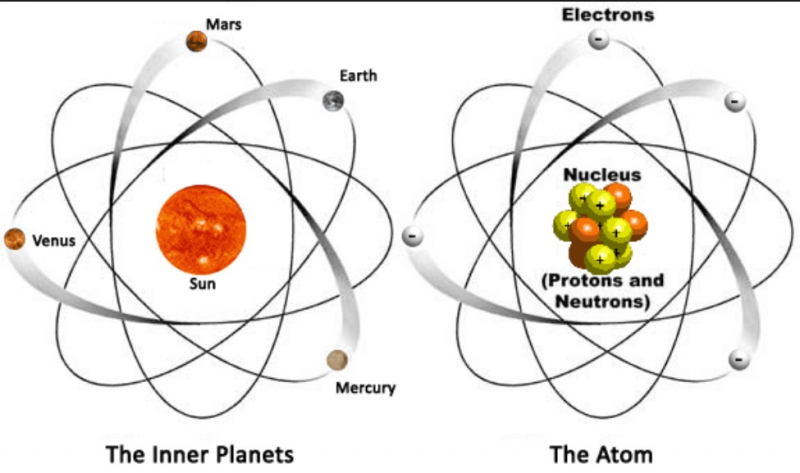
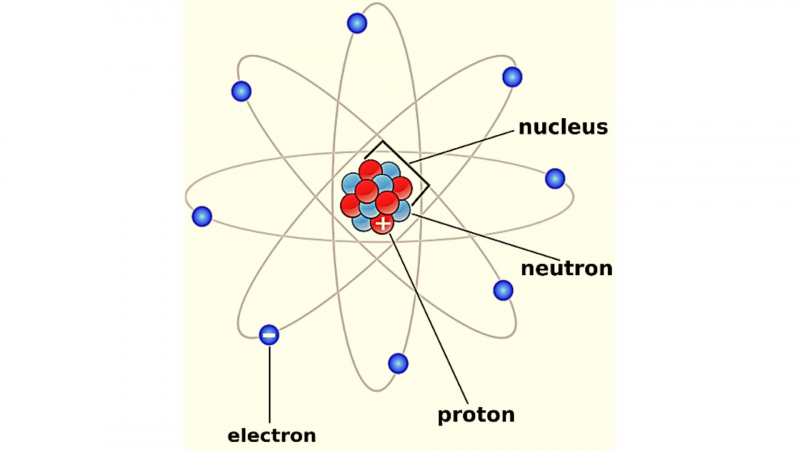
Albert Einstein Provided Empirical Evidence For The Atomic Theory
Robert Brown used a microscope to view pollen grains in water in 1827 and discovered that they moved through the water, but he was unable to determine the mechanisms that generated this mobility. Albert Einstein released a paper in 1905 on Brownian motion, which is the random motion of particles in a fluid. Brown had noticed motion in the pollen, which Einstein stated was caused by individual water molecules moving the pollen. Though scientists had long theorized about atoms and molecules, Einstein's explanation of Brownian motion served as irrefutable proof that atoms and molecules indeed exist.
According to atomic theory, any liquid is made up of molecules (invisible in 1905). Furthermore, these molecules are always moving in a random, unending manner. The overall qualities of any liquid are determined by the average behavior of these molecules. However, Einstein knew that the random chaos of jostling, unseen molecules would cause statistical fluctuations - a small group of invisible molecules could move in largely the same direction for a brief period of time. Then, for a brief moment, another neighboring set of molecules could migrate primarily in a different direction.