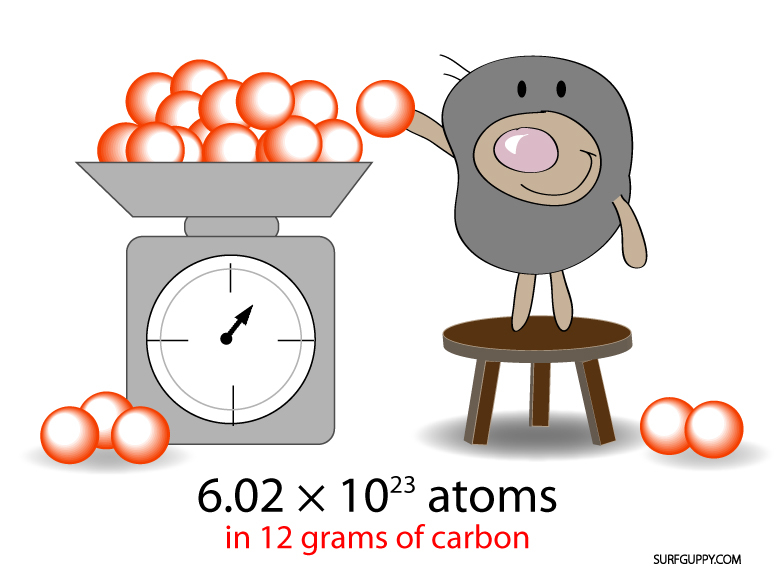
He Enabled The Determination Of Avogadro’s Number And The Size Of Molecules
One of the major accomplishments of Albert Einstein is Avogadro’s number. When explaining Brownian motion, Einstein established the size of atoms and the number of atoms in a mole. He made it possible to determine Avogadro's number, and thus the size of molecules, experimentally. Experimentalists were able to count atoms by staring through an ordinary microscope because of Einstein's statistical account of atomic activity.
The statistical study of the Brownian motion of oil particles dispersed in water was used by Einstein's approach. In a microscope, he determined the average distance of the droplets due to their random Brownian motion. It is dependent on the atom size. If the atoms are smaller, the Brownian motion will be slower due to greater statistical leveling of the water molecules around the oil drops.
The influence of a single molecule striking a crumb that is many orders of magnitude larger may be insignificant. The aggregate displacement from numerous such strikes (so-called Brownian motion), according to Einstein, may reach a quantifiable distance. He computed the distance by treating the specks' motion as "random walks," jolting around through impulses that are unexpected in size and direction, but with an overall distribution he determined for his thesis.