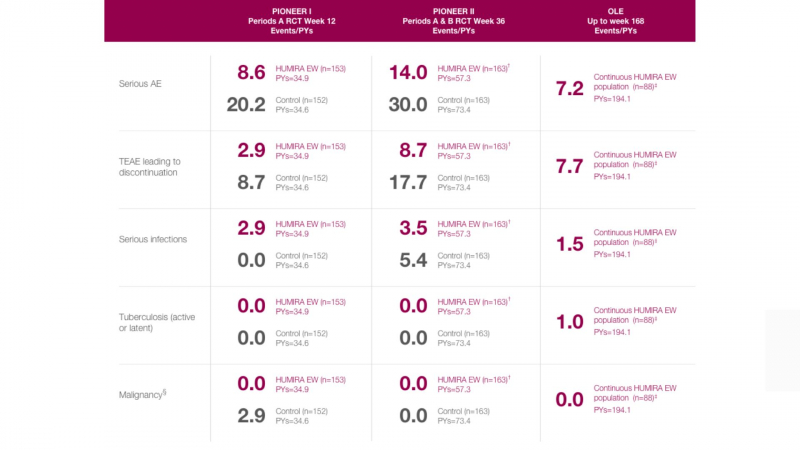
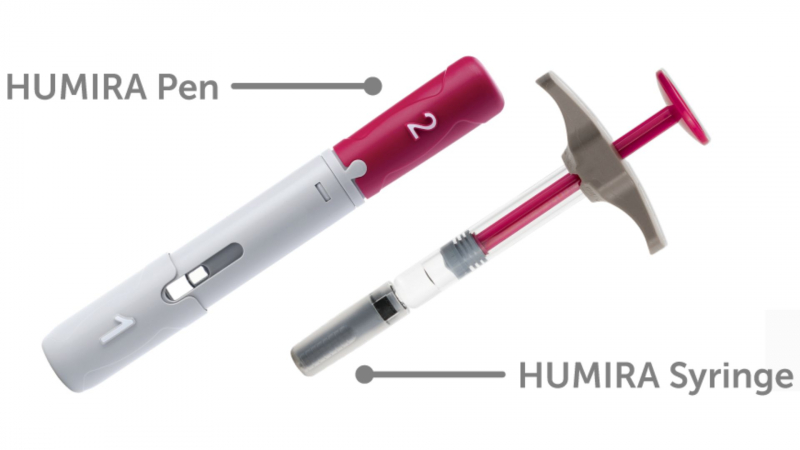
Dosage: How Much Humira Should I Take?

This medication's dosage will vary depending on the patient. Follow your doctor's orders or the label's instructions. This information only includes the average doses of this medication. If your dose differs, do not change it unless your doctor instructs you to.
The amount of medicine you take is determined by the strength of the medicine. In addition, the number of doses you take each day, the time between doses, and the length of time you take the medicine are all determined by the medical problem for which you are using the medicine.
For injection dosage forms (pen or prefilled syringe):
For Crohn's disease:
- Both adults and children 6 years of age and older weighing 40 kilograms (kg) or more—At first (week 0), 160 milligrams (mg) injected under the skin in divided doses. This can be administered in four shots in one day or two shots per day for two days. Then, two weeks later, a 80 mg dose is administered. A maintenance dose of 40 mg is administered at week 4 and every other week after that.
- Children 6 years of age and older weighing 17 kg but less than 40 kg—80 mg injected under the skin in divided doses at first (week 0). This can be administered as two shots in one day. Then, two weeks later, a dose of 40 mg is administered. A maintenance dose of 20 mg is administered at week 4 and every other week after that.
- Children under the age of six years old—Use and dosage must be determined by your doctor.
For hidradenitis suppurativa:
- Adults— 160 milligrams (mg) injected under the skin in divided doses at first (week 0). This can be administered in four shots in one day or two shots per day for two days. Then, two weeks later, an 80 mg dose is administered. Beginning in week 4, a maintenance dose of 40 mg every week or 80 mg every other week is administered.
- Children 12 and older weighing 60 kilograms (kg) or more—The dose is based on body weight and must be administered by your doctor. At the beginning (week 0), 160 mg was injected under the skin in divided doses. This can be administered in four shots in one day or two shots per day for two days. Then, two weeks later, an 80 mg dose is administered. Beginning in week 4, a maintenance dose of 40 mg every week or 80 mg every other week is administered.
- Children aged 12 and up weighing 30 kg to 59 kg—The dose is based on body weight and must be administered by your doctor. On Day 1, 80 mg was injected under the skin, followed by 40 mg on Day 8. Every other week, a maintenance dose of 40 mg is administered.
- Children under the age of 12 or weighing less than 30 kg—Use and dosage must be determined by your doctor.
For juvenile idiopathic arthritis:
- Children aged 2 to 17 weighing 30 kilograms (kg) or more—40 milligrams (mg) injected under the skin every other week.
- Children aged 2 to 17 weighing 15 to 30 kg—20 mg injected under the skin every other week.
- Children aged 2 to 17 weighing 10 to 15 kg—10 mg injected under the skin every other week.
- Children under the age of two or weighing less than ten kilograms—Use and dose must be determined by your doctor.
For plaque psoriasis:
- Adults—At first, 80 milligrams (mg) injected under the skin, then 40 mg 1 week later and every other week after that.
- Your doctor will determine the appropriate use and dosage for your child.
For psoriatic arthritis, rheumatoid arthritis, or ankylosing spondylitis:
- Adults—every other week, 40 milligrams (mg) injected under the skin. As needed, your doctor may adjust your dose. Some rheumatoid arthritis patients who are not taking methotrexate may take 40 mg every week or 80 mg every other week.
- Your doctor will determine the appropriate use and dosage for your child.
For ulcerative colitis:
- Adults—At first (week 0), 160 milligrams (mg) injected under the skin in divided doses. This may be given as four shots in 1 day or as two shots per day for 2 days. Then 2 weeks later, a dose of 80 mg is given. A maintenance dose of 40 mg is given at week 4 and every other week thereafter.
- Children 5 years of age and older weighing 40 kilograms (kg) or more—At first (Day 1), 160 mg injected under the skin as a single dose in 1 day or in divided doses. This may be given as four shots in 1 day or as two shots per day for 2 days. Then 80 mg is given on Day 8 and Day 15. A maintenance dose of 80 mg every other week or 40 mg every week is given starting at week 4 (Day 29).
- Children 5 years of age and older weighing 20 to less than 40 kg—At first (Day 1), 80 mg injected under the skin, then 40 mg on Day 8 and Day 15. A maintenance dose of 40 mg every other week or 20 mg every week is given starting at week 4 (Day 29).
- Children younger than 5 years of age—Use and dose must be determined by your doctor.
For uveitis:
- Adults—At first, 80 milligrams (mg) injected under the skin, then 40 mg 1 week after the initial dose and every other week thereafter.
- Children 2 to 17 years of age weighing 30 kilograms (kg) or more—40 milligrams (mg) injected under the skin every other week.
- Children 2 to 17 years of age weighing 15 to less than 30 kg—20 mg injected under the skin every other week.
- Children 2 to 17 years of age weighing 10 to less than 15 kg—10 mg injected under the skin every other week.
- Children younger than 2 years of age or weighing less than 10 kg—Use and dose must be determined by your doctor.
Modifications
The citrate-free formulations are used in prefilled pens or syringes. Citrate-free formulations may be less painful to inject.
Missed Dose
If you forget to take a dose, take it as soon as possible. If your next dose is too close, skip the missed dose. Return to your regular schedule. Do not inject two doses at once or use excessive amounts.