Top 9 Things to Know About Humira
Humira is a medication that helps relieve pain and inflammation for patients with chronic autoimmune arthritis. The use of the drug needs to be controlled and ... read more...closely monitored by a doctor, because there is a risk of many effects on the body. Here are the top things to know about Humira.
-
Humira (adalimumab) is an injectable medication used to treat Crohn's disease, plaque psoriasis, ulcerative colitis, and certain types of arthritis. It also treats uveitis and a skin condition known as hidradenitis suppurativa. Humira is available in prefilled syringes, prefilled pens, and vials.
Adalimumab is a type of monoclonal antibody. It inhibits the activity of tumor necrosis factor (TNF) molecule.
TNF molecules are produced by the immune system and aid in the fight against germs. These molecules cause swelling when your body fights germs. TNF molecules are sometimes produced in excess by the body. Excess molecules cause pain and damage, as well as inflammatory diseases.
Humira binds to and inhibits TNF molecules. Adalimumab reduces swelling and pain by blocking TNF molecules. It alleviates symptoms as well as the damage caused by inflammatory conditions.

NiceRx 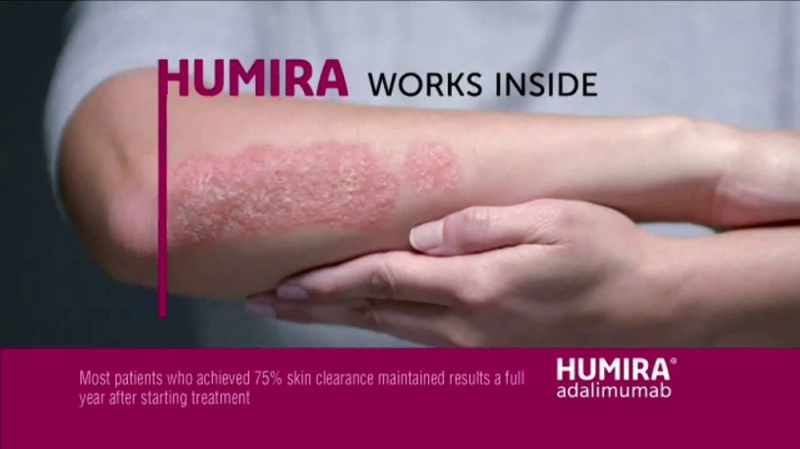
iSpot.tv -
The Food and Drug Administration (FDA) approved Humira to treat the following:
- Crohn’s disease (inflammatory bowel disease, or IBD, affecting your digestive tract)
- Ulcerative colitis (IBD of the large intestine)
- Plaque psoriasis (autoimmune disease causing thick, red, scaly skin patches)
- Psoriatic arthritis (autoimmune disease that is a type of inflammatory arthritis)
- Rheumatoid arthritis (autoimmune disease causing painful, swollen, and stiff joints)
- Juvenile idiopathic arthritis (chronic arthritis in children and teens)
- Uveitis (eye inflammation)
- Ankylosing spondylitis (autoimmune disease causing bones in the spine to fuse)
- Hidradenitis suppurativa (chronic skin condition causing painful lumps)
The FDA has issued a boxed warning for Humira regarding an increased risk of severe infections and cancers such as lymphoma, a cancer of the lymphatic system, a network that aids in disease-fighting. A boxed warning is one of the FDA's most severe warnings about a drug's side effects. If you are concerned about using this medication, speak with your doctor.
How to Take Humira
Humira can be given to you every two weeks using prefilled pens and syringes. A healthcare provider will demonstrate how to use it.
Take Humira out of the refrigerator and let it sit at room temperature for 15-30 minutes before using it. This reduces the pain of the injection. While waiting for your medication to reach room temperature, do not heat it up or remove the cap or cover. Latex may be present in the needle cover for the pen and the cap of the prefilled syringe.
Before administering the medication, examine its appearance: Do not use the liquid if it is cloudy, colored, or leaking. Hands should be washed before and after use.To administer:
- As directed by your healthcare provider, inject the medication under your skin (subcutaneously).
- Inject at least 2 inches from your naval into the fatty part of the skin on top of your lower stomach or thigh.
- Ensure that injection sites are rotated.
Inject into the skin that is hard, bruised, red, or tender. In addition, avoid injecting in areas of the skin with stretch marks, psoriasis plaques, or scars.
Humira vials are only intended for institutional use. They do not contain any preservatives. Throw away any leftovers after using.
Needles and syringes should not be reused. Instead, place used needles or syringes in a sharps (needle) container and dispose of full containers in accordance with local regulations. If you have any questions, consult your doctor or pharmacist.Storage
Humira should be kept in the refrigerator but not frozen. If the medication freezes, do not use it again until it has thawed. Adalimumab can be stored at room temperature.
After removing it from the refrigerator, make a note of the date. Toss it 14 days after taking it out of the fridge. It should not be used after 14 days.
Keep Humira away from heat and light, and keep it out of the reach of children and pets. Throw away any leftovers.
Do not flush your medication down the toilet or drain. Find out where your local drug disposal sites are. On the FDA website, you can look for programs in your area. If you have any questions, please contact your pharmacist, who will be able to advise you on proper disposal.Off-Label Uses
Healthcare providers occasionally prescribe Humira for purposes not approved by the FDA. This is referred to as off-label use. Although not approved for these conditions, Humira may be beneficial in treating them under the supervision of your doctor.
Off-label use for Humira are:
- Crohn's disease treatment after removing all or a portion of the affected organ
- Management of a severe form of Crohn's disease known as perianal/fistulizing disease
- Pyoderma gangrenosum (large painful sores on the skin)
- Alopecia areata (sudden hair loss with overlapping bald patches)

Verywell Health 
CreakyJoints -
Humira, like other medications, can have side effects. Inform your doctor about any side effects you experience while taking this medication.
Common Side Effects
Humira commonly causes the following side effects:
- Back pain
- Headache
- Nausea
- Stomach pain
- Irritation where the shot is given
- Signs of a common cold
If any of these side effects worsen or do not go away, contact your healthcare provider.
Severe Side Effects
If you experience severe side effects, contact your doctor right away. If your symptoms are life-threatening or you believe you have a medical emergency, dial 911. The following are examples of serious side effects and symptoms:
Signs of a urinary tract infection (UTI):
- Fever
- Blood in the urine
- Lower belly pain
- Feeling the need to pass urine many times or right away
- Burning or pain when passing urine
Signs of high blood pressure:
- Dizziness
- Terrible headache
- Change in vision
- Passing out
Signs of an allergic reaction:
- Hives
- Rash
- Wheezing
- Itching
- Peeling skin, with or without fever
- Tightness in the chest
- Trouble breathing, swallowing, or talking
- Swelling of the tongue, face, throat, mouth, or lips
Signs of lupus (autoimmune condition causing inflammation of the joints, skin, and other organs):
- Muscle pain
- Joint pain
- Sunburn easily
- Rash on the cheek
- Swelling in the arms or legs
Signs of infection:
- Cough
- Mouth sores
- Fever
- Ear pain
- Wounds that will not heal
- Change in color of sputum
- Pain with urinating
Signs of liver problems:
- Dark urine
- Pale skin
- Fever
- Easy bruising
Other severe side effects:
- A growth or lump on the skin
- Red bumps and patches filled with pus
- Weight loss without trying
- Night sweats (extreme perspiration as you sleep)
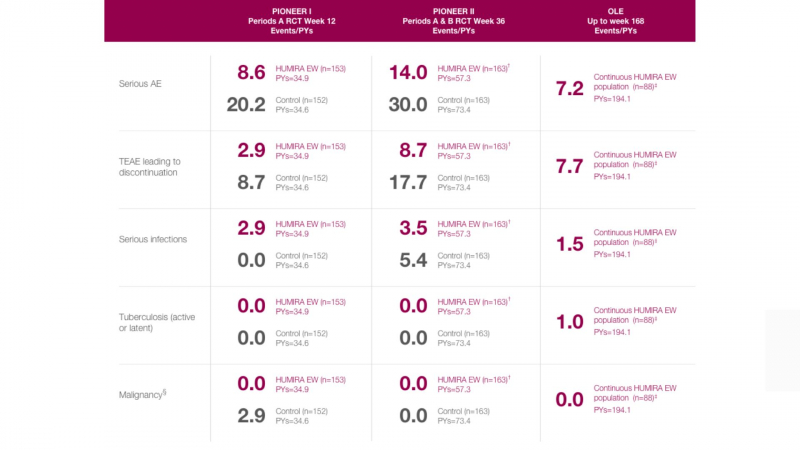
Humira comes with a boxed warning about the increased risk of serious infections, such as tuberculosis (TB, a lung disease), bacterial sepsis (a serious, life-threatening reaction to an infection), and invasive fungal infections. Before starting Humira, your healthcare provider may test you for latent tuberculosis (TB bacteria in your body that is not active) and monitor you for active TB while you are taking it.
The FDA also warns about the risk of lymphoma and other cancers developing in children and adolescents who take TNF blockers like Humira.
Apollo Hospitals Blog - AskApollo 
Cleveland Clinic Health Essentials -
This medication's dosage will vary depending on the patient. Follow your doctor's orders or the label's instructions. This information only includes the average doses of this medication. If your dose differs, do not change it unless your doctor instructs you to.
The amount of medicine you take is determined by the strength of the medicine. In addition, the number of doses you take each day, the time between doses, and the length of time you take the medicine are all determined by the medical problem for which you are using the medicine.
For injection dosage forms (pen or prefilled syringe):
For Crohn's disease:
- Both adults and children 6 years of age and older weighing 40 kilograms (kg) or more—At first (week 0), 160 milligrams (mg) injected under the skin in divided doses. This can be administered in four shots in one day or two shots per day for two days. Then, two weeks later, a 80 mg dose is administered. A maintenance dose of 40 mg is administered at week 4 and every other week after that.
- Children 6 years of age and older weighing 17 kg but less than 40 kg—80 mg injected under the skin in divided doses at first (week 0). This can be administered as two shots in one day. Then, two weeks later, a dose of 40 mg is administered. A maintenance dose of 20 mg is administered at week 4 and every other week after that.
- Children under the age of six years old—Use and dosage must be determined by your doctor.
For hidradenitis suppurativa:
- Adults— 160 milligrams (mg) injected under the skin in divided doses at first (week 0). This can be administered in four shots in one day or two shots per day for two days. Then, two weeks later, an 80 mg dose is administered. Beginning in week 4, a maintenance dose of 40 mg every week or 80 mg every other week is administered.
- Children 12 and older weighing 60 kilograms (kg) or more—The dose is based on body weight and must be administered by your doctor. At the beginning (week 0), 160 mg was injected under the skin in divided doses. This can be administered in four shots in one day or two shots per day for two days. Then, two weeks later, an 80 mg dose is administered. Beginning in week 4, a maintenance dose of 40 mg every week or 80 mg every other week is administered.
- Children aged 12 and up weighing 30 kg to 59 kg—The dose is based on body weight and must be administered by your doctor. On Day 1, 80 mg was injected under the skin, followed by 40 mg on Day 8. Every other week, a maintenance dose of 40 mg is administered.
- Children under the age of 12 or weighing less than 30 kg—Use and dosage must be determined by your doctor.
For juvenile idiopathic arthritis:
- Children aged 2 to 17 weighing 30 kilograms (kg) or more—40 milligrams (mg) injected under the skin every other week.
- Children aged 2 to 17 weighing 15 to 30 kg—20 mg injected under the skin every other week.
- Children aged 2 to 17 weighing 10 to 15 kg—10 mg injected under the skin every other week.
- Children under the age of two or weighing less than ten kilograms—Use and dose must be determined by your doctor.
For plaque psoriasis:
- Adults—At first, 80 milligrams (mg) injected under the skin, then 40 mg 1 week later and every other week after that.
- Your doctor will determine the appropriate use and dosage for your child.
For psoriatic arthritis, rheumatoid arthritis, or ankylosing spondylitis:
- Adults—every other week, 40 milligrams (mg) injected under the skin. As needed, your doctor may adjust your dose. Some rheumatoid arthritis patients who are not taking methotrexate may take 40 mg every week or 80 mg every other week.
- Your doctor will determine the appropriate use and dosage for your child.
For ulcerative colitis:
- Adults—At first (week 0), 160 milligrams (mg) injected under the skin in divided doses. This may be given as four shots in 1 day or as two shots per day for 2 days. Then 2 weeks later, a dose of 80 mg is given. A maintenance dose of 40 mg is given at week 4 and every other week thereafter.
- Children 5 years of age and older weighing 40 kilograms (kg) or more—At first (Day 1), 160 mg injected under the skin as a single dose in 1 day or in divided doses. This may be given as four shots in 1 day or as two shots per day for 2 days. Then 80 mg is given on Day 8 and Day 15. A maintenance dose of 80 mg every other week or 40 mg every week is given starting at week 4 (Day 29).
- Children 5 years of age and older weighing 20 to less than 40 kg—At first (Day 1), 80 mg injected under the skin, then 40 mg on Day 8 and Day 15. A maintenance dose of 40 mg every other week or 20 mg every week is given starting at week 4 (Day 29).
- Children younger than 5 years of age—Use and dose must be determined by your doctor.
For uveitis:
- Adults—At first, 80 milligrams (mg) injected under the skin, then 40 mg 1 week after the initial dose and every other week thereafter.
- Children 2 to 17 years of age weighing 30 kilograms (kg) or more—40 milligrams (mg) injected under the skin every other week.
- Children 2 to 17 years of age weighing 15 to less than 30 kg—20 mg injected under the skin every other week.
- Children 2 to 17 years of age weighing 10 to less than 15 kg—10 mg injected under the skin every other week.
- Children younger than 2 years of age or weighing less than 10 kg—Use and dose must be determined by your doctor.
Modifications
The citrate-free formulations are used in prefilled pens or syringes. Citrate-free formulations may be less painful to inject.
Missed Dose
If you forget to take a dose, take it as soon as possible. If your next dose is too close, skip the missed dose. Return to your regular schedule. Do not inject two doses at once or use excessive amounts.
www.humiradermpro.com 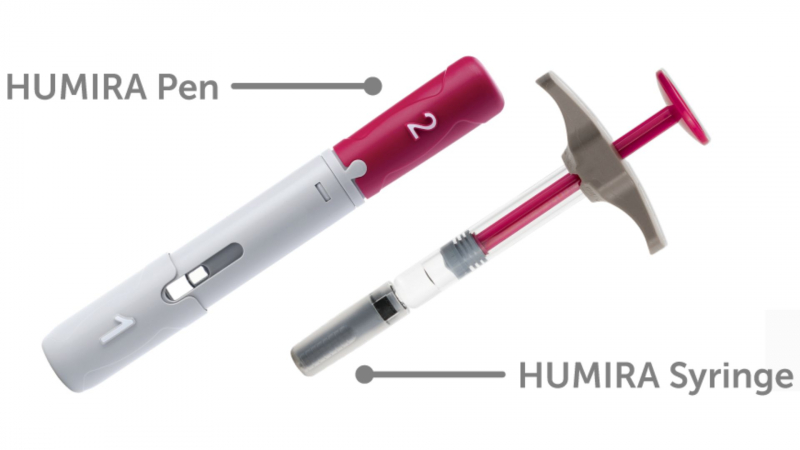
Humira -
Do not exceed the recommended dosage of Humira. In the event of an overdose, you may require monitoring for signs of adverse reactions or side effects.
If you believe you or someone else has overdosed on Humira, contact your doctor or the Poison Control Center (1-800-222-1222) right away.
Call 911 immediately if someone collapses or stops breathing after taking Humira.
American Association of Poison Control Centers 
Walden University -
If you will be taking this medication for an extended period of time, it is critical that your doctor monitor your or your child's progress on a regular basis. This will allow your doctor to determine whether or not the medication is working properly and whether or not you should continue to take it. To rule out any negative effects, blood and urine tests may be required.
Before using this medication, you or your child must have a tuberculosis skin test. Inform your doctor if you or anyone in your household has ever had a positive tuberculosis skin test reaction.
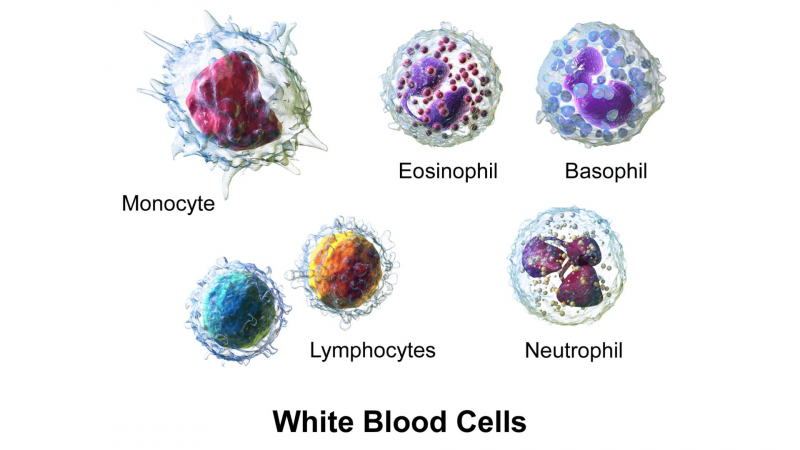
Adalimumab can temporarily reduce the number of white blood cells in your blood, increasing your risk of infection. It can also reduce the number of platelets, which are required for blood clotting. If this happens, there are some precautions you can take to reduce the risk of infection or bleeding, especially if your blood count is low:
- Avoid infected people if possible. If you or your child suspects an infection or if you experience fever or chills, cough or hoarseness, lower back or side pain, or painful or difficult urination, consult your doctor right away.
If you or your child notice any unusual bleeding or bruising, black, tarry stools, blood in the urine or stools, or pinpoint red spots on your skin, contact your doctor right away.
When using a regular toothbrush, dental floss, or toothpick, use caution. Other methods of cleaning your teeth and gums may be suggested by your doctor, dentist, or nurse. Before having any dental work done, consult with your doctor. - Touch your eyes or the inside of your nose only after you've washed your hands and haven't touched anything else in the meantime.
When using sharp objects such as a safety razor or fingernail or toenail cutters, take care not to cut yourself.
Avoid contact sports and other situations that could result in bruising or injury.
Other medications should not be taken unless they have been discussed with your doctor. Combining this medication with abatacept (Orencia®) or anakinra (Kineret®) may increase your risk of serious side effects.
This medication may cause other side effects that do not appear for months or years after it is taken. A small number of people (including children and teenagers) who used this type of medicine developed cancer (eg, leukemia). Some patients also developed lymphoma, a rare type of cancer. If you or your child experience unusual bleeding, bruising, or weakness, swollen lymph nodes in the neck, underarms, groin, or unexplained weight loss, consult your doctor. Also, if your or your child's skin has red, scaly patches or raised bumps filled with pus, consult your doctor right away.
Adalimumab can cause severe allergic reactions (such as anaphylaxis and angioneurotic edema), which can be fatal and necessitate immediate medical attention. If you or your child develops a cough, difficulty swallowing, dizziness, fast heartbeat, large, hive-like swelling on the face, eyelids, lips, tongue, throat, hands, legs, feet, sex organs, rash, itching, trouble breathing, or unusual tiredness or weakness after receiving the medication, contact your doctor right away.
If you or your child has sudden weight gain or swelling of the face, fingers, feet, or lower legs, consult your doctor right away. These could be symptoms of a heart condition known as congestive heart failure (CHF).Some people who took this medication experienced lupus-like symptoms during treatment and recovered once the medication was stopped. Consult your doctor right away if you or your child develops chest pains, joint pain, or a sun-sensitive rash on the cheeks or arms.
While you or your child is receiving adalimumab treatment, do not receive any live vaccines (immunizations). Before using adalimumab, your child's vaccines must be up to date. If you have any concerns, consult with your child's doctor.Some prefilled syringes and pens have dry natural rubber (a latex derivative) in the needle cap, which can make some people allergic to latex. Before taking this medication, let your doctor know if you or your child have a latex allergy.
When taking this medication, serious skin reactions can happen. If you or your child experiences blistering, peeling, or loosening of the skin, red skin lesions, severe acne or skin rash, sores or ulcers on the skin, fever, or chills while taking this medication, consult your doctor right away.What Are Reasons I Shouldn't Take Humira?
In most circumstances, using Humira is safe. There are some circumstances, though, in which your doctor might not recommend Humira for you.
Heart failure is one condition that Humira can make worse. As a result, you might not be able to use this medication if you have heart failure. Additionally, it can raise your risk of contracting serious infections like TB, which can be fatal or require hospitalization.
Humira should also be avoided if you have:
- Liver problems
- An allergy or hypersensitivity to it
Humira is not prescribed to children under the age of 4 or weighing less than 11 pounds as a precaution. This group lacks sufficient data.
SelfDecode Labs 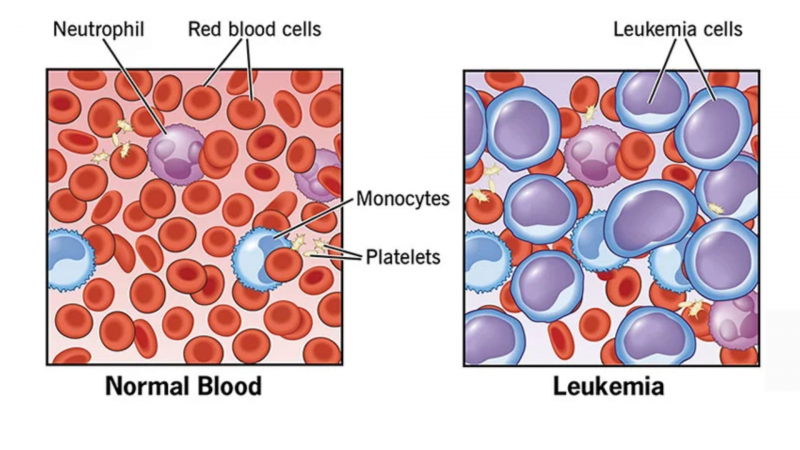
Cleveland Clinic - Avoid infected people if possible. If you or your child suspects an infection or if you experience fever or chills, cough or hoarseness, lower back or side pain, or painful or difficult urination, consult your doctor right away.
-
Humira may interact with certain medications. These interactions can either increase or decrease the effectiveness of your treatment.
Humira can interact with the following substances:
- Echinacea
- Live vaccines
- Arava (leflunomide)
Echinacea
Echinacea is a supplement that is available over-the-counter (OTC). It has numerous health benefits, but it may reduce the effectiveness of adalimumab. Do not take echinacea without first consulting your doctor.
Live Vaccines
Humira can exacerbate the adverse effects of live vaccines. It may also have an impact on how well a live vaccine works. Do not receive live vaccines if you are taking adalimumab. Before getting immunized, you should wait at least three months after taking Humira. MMR (measles, mumps, rubella), varicella (chickenpox), rotavirus (which causes severe diarrhea), and the intranasal flu vaccine are all examples of live vaccines.
Arava (Leflunomide)
Arava is used to treat conditions such as rheumatoid arthritis. When combined with Arava, Humira may exacerbate its side effects.
This combination can cause blood problems, such as:- Pancytopenia (low red and white blood cells and low platelet count)
- Agranulocytosis (the body does not make white blood cells)
- Thrombocytopenia (low platelet level).
If you are taking both drugs, your healthcare provider should monitor your bone marrow at least monthly.

iStock 
Pew Research Center -
Humira is a biologic disease-modifying antirheumatic drug (DMARD).
These medications have an effect on the immune system. Using DMARDs in conjunction with Humira will reduce your immune system function even further. You should avoid taking these medications while taking adalimumab.
Examples of DMARDs include:
- Remicade (infliximab)
- Truxima (rituximab)
- Enbrel (etanercept)
- Orencia (abatacept)
- Actemra (tocilizumab)
- Xeljanz(tofacitinib)
Everyday Health 
بهستان دارو -
Humira weakens your immune system. Be aware of this and take precautions to protect yourself from germs. Always wash your hands before and after taking your medication.
Before taking another OTC drug, supplement, or herb, always consult with your healthcare provider. Do not start, stop, or adjust your medication without first consulting your provider. Humira may cause you to bleed more easily. Avoid getting hurt while taking this medication. Use a soft toothbrush and an electric razor to keep your gums and skin from bleeding.
Joe DiMaggio Children's Hospital 
Adobe Stock