John Dalton Is Most Famous For His Atomic Theory
The Atomic Theory was Dalton's most famous contribution to science. Democritus (460–370 B.C.E.), a Greek philosopher, postulated that all substance was made up of tiny particles called atoms, which were invisible to the naked eye.
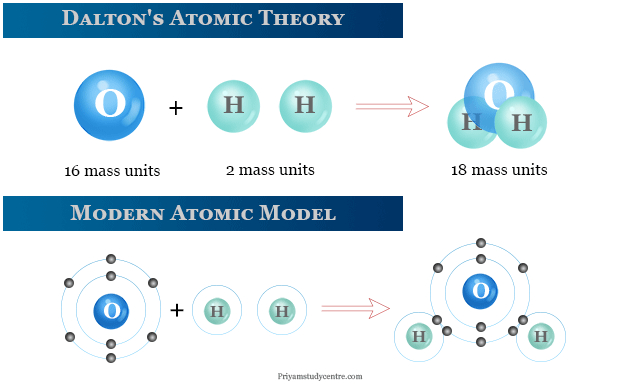
Dalton disagreed with the widespread belief at the time that all atoms in all kinds of matter were the same. Instead, he proposed that all atoms of a given element were the same, but that atoms of different elements differed in size and mass. He went on to say that more complicated compounds were generated by combining two or more distinct atoms, and that chemical reactions were nothing more than atom rearrangements. He came up with The Law of Multiple Proportions, which states that when atoms join to form compounds, the weight ratio is always a whole number. When a fixed amount of one element is coupled with a second element of uncertain mass, the mass ratios will be small whole numbers as well.
Dalton backed up his hypothesis with experiments, calculating the relative weight of atoms by observing how elements like hydrogen (to which he assigned an arbitrary weight of 1) mixed with fixed proportions of other elements. In a series of articles, he presented his atomic hypothesis to the Manchester society in 1803. He presented his findings in the two-volume book, New System of Chemical Philosophy, after being encouraged by his peers in the organization (part one in 1808 and part two in 1810). Despite issues with his atomic weight measurement, his idea was fundamentally sound and eventually became a part of modern chemistry's foundation.