Tips

Top 5 in Top 7 Things to Know About Brilinta
Brilinta is an oral tablet that is typically taken twice a day, in the morning and at night. Brilinta can be taken with or without food and is usually combined with a daily aspirin maintenance dose. Brilinta's first dose may be higher than subsequent doses. This is known as a loading dose, and it allows Brilinta to reach effective levels more quickly.
Brilinta should not be abruptly discontinued without first consulting your cardiologist. Brilinta can increase the risk of a heart attack, stroke, or death if it is stopped too soon. If you are going to have surgery, your doctor may tell you to stop taking Brilinta 5 days before the procedure. This will help to reduce the likelihood of bleeding. Follow your doctor's instructions for stopping and restarting Brilinta.
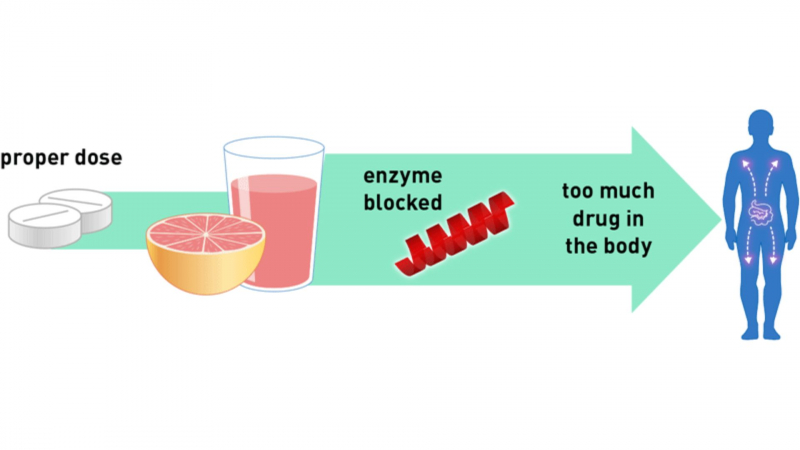
While taking Brilinta, avoid drinking grapefruit juice. Grapefruit juice has the ability to inhibit (block) one of the enzymes (3A4) required for the breakdown of Brilinta for excretion from the body. In theory, drinking grapefruit juice while taking Brilinta could increase the antiplatelet effect of your medication and increase your risk of bleeding. Inquire with your doctor about this potential drug-food interaction.
Brilinta does not have the same dietary restrictions as warfarin, so you can eat green leafy vegetables like spinach, broccoli, kale, and other vitamin K-rich foods while taking Brilinta.
It is not advisable to consume alcohol while taking Brilinta because you will also be taking aspirin. Aspirin combined with alcohol can cause stomach bleeding and ulcers. Inform your doctor if you have a history of stomach ulcers or digestive tract bleeding. If you have symptoms of bleeding in your stomach or intestines, contact your doctor right away. This includes black, bloody, or tarry stools, as well as coughing up blood or vomit resembling coffee grounds.
Do not take any other medications, including those purchased at a supermarket or drug store, without first consulting your doctor or a pharmacist. It is safe to take acetaminophen (Tylenol) with Brilinta if you need a mild pain reliever. Many over-the-counter (OTC) products contain aspirin, ibuprofen (Advil, Motrin), or naproxen (Aleve), which are incompatible with Brilinta. These ingredients are also found in combination products used to treat cold or flu symptoms or to help you sleep. Check the labels to see if these ingredients are present. If you are unsure, consult your pharmacist.
While taking Brilinta, you may notice that you bruise or bleed more easily and that it takes longer to stop bleeding. This demonstrates Brilinta's productivity. If the bleeding is excessive or prolonged, or if you notice blood in your urine or stool, seek immediate medical attention.
Seek medical attention right away if you develop a fever, weakness, skin or eye yellowing, feel confused, or your skin appears extremely pale.
Inform your dentist and other health care providers that you are taking Brilinta before any invasive procedure.
Although there was no evidence of an increased risk of major birth defects in case studies investigating Brilinta use during pregnancy, animal studies using more than the maximum recommended dose did show an increased risk of structural abnormalities, pup death, and growth delays. If you are taking Brilinta, you should notify your doctor if you are planning a pregnancy or if you become pregnant unintentionally. Breastfeeding is not advised during Brilinta treatment.