Top 7 Things to Know About Dupixent
Dupixent is indicated for the treatment of adults and children aged 6 months and older with moderate-to-severe eczema (atopic dermatitis) whose disease is not ... read more...adequately controlled with topical prescription therapies or when those therapies are not advisable. Dupixent can be used with or without topical corticosteroids.
-
Dupilumab is sold under the trade name Dupixent and is sometimes used to treat eczema, asthma, and other disorders that cause inflammation.
By attaching to the interleukin-4R subunit, which is shared by the interleukin-4 (IL-4) and IL-13 receptor complexes, dupixent (dupilumab) inhibits the inflammatory response. Interleukins are cytokines that are produced spontaneously. Immune system cells produce cytokines, which have an impact on other cells. Many immune system cell types, including mast cells, eosinophils, macrophages, lymphocytes, epithelial cells, and goblet cells, express IL-4R. These cause the release of chemicals that contribute to inflammation, including histamine, eicosanoids, cytokines, etc. Dupixent, which blocks IL-4R, reduces the inflammatory response brought on by IL-4 and IL-13 in conditions like eczema, nasal polyps, and eosinophilic esophagitis. Dupixent's precise method of action in asthma is unclear.
The group of drugs known as interleukin inhibitors includes dupixent. It can also be referred to as a biologic or a human monoclonal antibody.

dupixent.com 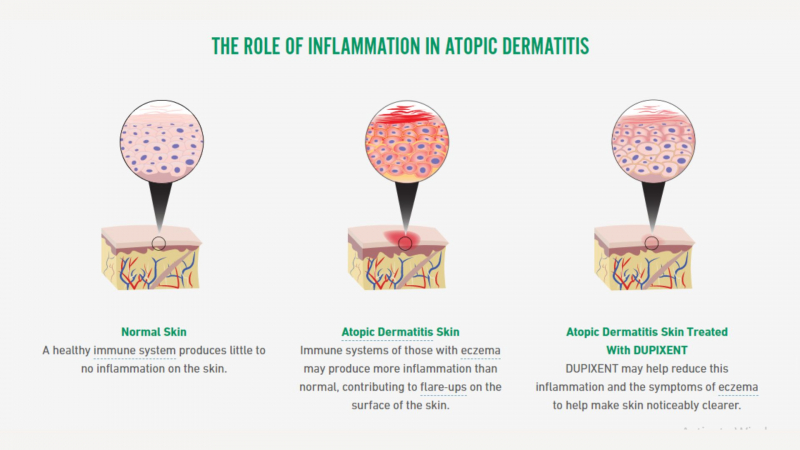
dupixent.com -
When other topical therapies are ineffective or unsafe, it may be used to treat moderate-to-severe eczema (atopic dermatitis) in adults and children aged 6 months and older. It is safe to use with or without topical corticosteroids.
As a maintenance add-on therapy for adults and children 6 years of age and older, it may also be used for moderate-to-severe eosinophilic asthma (a condition marked by elevated levels of the white blood cells known as eosinophils). A flare-up of asthma is NOT treated with dupixent.
Can be used in addition to other treatments for chronic rhinosinusitis with nasal polyps in adults and eosinophilic esophagitis in adults and children aged 12 and up who weigh at least 40 kg.
Is the first (and currently only) approved treatment for Prurigo Nodularis, a chronic, debilitating skin disease that has a significant impact on a person's quality of life.
Steroid-free.
Subcutaneous injection is used to administer the medication (under the skin).
Pre-filled syringes and pre-filled pens are available. The pre-filled pen is only for patients over the age of 12. The pre-filled pen contains a dose of 300 mg. The pre-filled syringe is available in 200 mg or 300 mg doses.
Dupixent patients can be taught how to self-administer the injection.
Usually administered every two weeks (every other week). Dupixent should be given every four weeks to children weighing 15kg to less than 30kg.
Because it does not suppress the immune system, it is not classified as an immunosuppressant.
Although it is not a cure for eczema, it does reduce symptoms (such as itching, rash, and flare frequency) in two-thirds to three-fourths of those who take it. However, there appears to be a subset of people who only respond partially to Dupixent (partial responders), or who respond initially and then have their symptoms return (non-durable responders).
eMPR.com 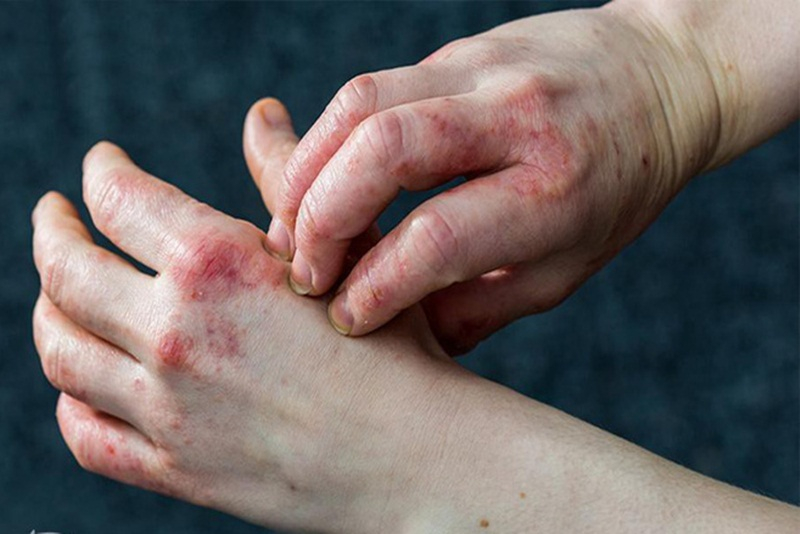
Medlatec.vn -
If you are between the ages of 18 and 60, do not take any other medications, and have no other medical conditions, you are more likely to experience the following side effects:
- The most common side effects reported are injection site reactions (such as redness, pain, or swelling at the injection site), conjunctivitis and other eye problems (such as dry eyes, itchy eyes, or keratitis), and herpes (eg, cold sores).
- Although weight gain is not listed as a side effect in the product information, a published case series comparing twelve people prescribed Dupixent to eight people prescribed methotrexate reported significant weight gain in the Dupixent group (an average of 6.1kg over one year; the amount of weight gained ranged from 0.1kg to 18.0kg) compared to no weight gain or loss in the methotrexate group. People posting on blogs have anecdotally reported weight gain as a side effect.
- There have been numerous post-marketing reports of Dupixent-related tendinitis, arthritic-like discomfort, and aching joints. Joint pain brought on by Dupixent may be treated with naproxen, and if that doesn't work, methotrexate. If the joint discomfort is especially severe or persists despite treatment, it should be thought about stopping Dupixent.
- Like other biologic drugs, dupixent needs to be checked for efficiency and side effects. A new biologic or the inclusion of other therapies may be necessary in the event of a lack of response, loss of response, or significant adverse effects. When Dupixent is started, corticosteroids shouldn't be abruptly stopped; instead, they should be reduced gradually and done so under a doctor's supervision.
- Keep refrigerated between 2°C and 8°C [36°F to 46°F]). Protect from light by storing in the original carton. For up to 14 days, store at room temperature of up to 77ºF (25ºC). Shake, heat, freeze, or expose the pens to direct sunlight. If stored at room temperature, do not refrigerate and discard Dupixent if not used within 14 days.
- Before beginning Dupixent treatment, make sure that all patients have received all of the appropriate immunizations for their age, as recommended by national guidelines. It is critical that people who have been given Dupixent do not receive live vaccines.
- Less than 1% of persons who received Dupixent reported experiencing serious hypersensitivity events (such serum sickness and urticaria). If a reaction happens, stop taking Dupixent and start the proper treatment. Additionally, immunogenicity and the production of antibodies are possible. About 5% of patients receiving Dupixent up to week 52 developed antibodies against it; 2% of these antibodies were deemed neutralizing.
- It is unknown how Dupixent affects the developing fetus during pregnancy, but it is known that human IgG antibodies pass across the placental barrier. Examine the advantages of Dupixent treatment versus the hazards of uncontrolled disease. Call 1-877-311-8972 to sign up for the prenatal exposure registry if a woman becomes pregnant while taking Dupixent. On the impact of Dupixent during lactation, there are no data.
- There is no generic version of Dupixent available.
Seniors and children, people with certain medical conditions (such as liver or kidney problems, heart disease, diabetes, seizures), or people who take other medications are more likely to experience a broader range of side effects.

NiceRx 
Dupixent -
Every other week, Dupixent is often injected beneath the skin to treat eczema, asthma, and a few other inflammatory diseases. The most frequently reported side effects were injection site responses and eye problems, and unlike with many other biologics, there was little chance of infection.
Dupixent 
اليوم السابع -
Dupixent is available as a prefilled pen or a single-use prefilled syringe (with a needle shield). It is delivered as a subcutaneous (under the skin) injection and comes in a carton with two pens or syringes in each packet.
Ordinarily, Dupixent is given every other week (every second week).
Do not attempt to self-administer Dupixent until a healthcare professional has shown you how. Adults should supervise children aged 12 and up. Dupixent should be administered by an adult parent or caregiver to children under the age of 12. Except for the 2 inches (5 cm) around the belly button, the injection can be given into the thigh or stomach. If a caregiver injects the medication, the outer area of the upper arm can also be used. For each injection, select a different injection site. Dupixent should not be injected into skin that is tender, damaged, bruised, or scarred.
Use an alcohol wipe to clean the injection site. Before injecting, let your skin to dry. Get rid of the green cap. The pen should be held so you can view the window. At around a 90-degree angle from your skin, place the yellow needle cover there. The pre-filled pen should be pressed firmly against your skin until the yellow needle cover is hidden. When the injection begins, there will be a "click," and the window will begin to become yellow. Watch the window while continuing to push the pen against your skin. You'll see a total yellowing of the glass and a second "click." To ensure you receive the entire dose, keep pushing the pen against your skin while counting to five. Pull straight up after finishing your injection to remove the pen from the skin. The needle will be protected by the yellow needle cover. If there is any blood at the site, dab it lightly with a cotton ball or gauze pad. After the injection, do not rub your skin. Remove the pen and contact your healthcare provider if the window does not turn completely yellow. Do not take a second dose without first consulting with your healthcare provider. After using a pen, immediately place it in an FDA-approved sharps disposal container. Do not dispose of the pens in the trash.
Refrigerate unused Dupixent Pre-filled Pens between 36ºF and 46ºF (2ºC and 8ºC). Protect from light by storing in the original carton. Remove Dupixent from the refrigerator 30 to 45 minutes prior to injection and place it on a bench to warm up to room temperature. Dupixent can be stored at room temperature (77ºF [25ºC]) for up to 14 days if necessary. Shake, heat, freeze, or expose the pens to direct sunlight. Keep all medicines, including Dupixent Pre-filled Pens, out of the reach of children.
Side effects of Dupixent include injection site reactions, eye problems (such as conjunctivitis or eye inflammation), and cold sores on the lips and mouth (oral herpes); however, most of these side effects can be effectively managed with other treatments, such as eye drops or anti-virals, allowing people to continue using Dupixent, especially if it provides significant benefits.
Conjunctivitis, blepharitis, dry eyes, itchy eyes, and keratitis are all associated with this condition. The reason for this is that Dupixent works by inhibiting two inflammatory proteins, IL-4 and IL-13. IL-13 also stimulates goblet cells, which are responsible for producing mucus in the eye and ensuring the stability of tears. So, when Dupixent inhibits IL-13, it reduces the number of epithelial goblet cells, resulting in inflammation and eye problems. Most of the time, the inflammation is mild, but Dupixent may need to be discontinued in some patients. The majority of eye problems respond to standard treatments (eg, ophthalmic antibiotics for bacterial conjunctivitis).
If you feel facial or throat stiffness after taking Dupixent, please seek immediate medical treatment. If you develop any additional side effects, such as eye issues, herpes infections, or weight gain, let your doctor know.
Inform other medical providers that Dupixent is being administered to you. You should have had all necessary vaccines before beginning Dupixent. You should avoid obtaining any live vaccines while using Dupixent (such as the MMR vaccine or the chickenpox vaccine).
Although it is unknown how Dupixent affects a developing infant, your doctor may still recommend using it if the advantages outweigh the dangers. If you become pregnant while taking Dupixent, tell your doctor right away.

Verywell Health 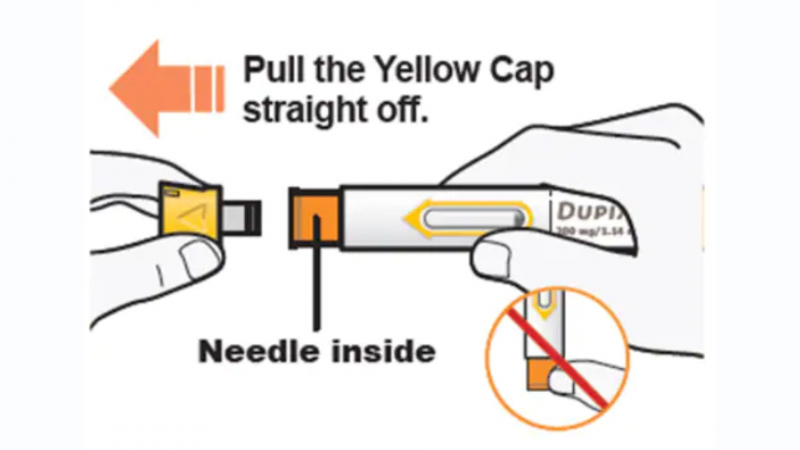
Drugs.com -
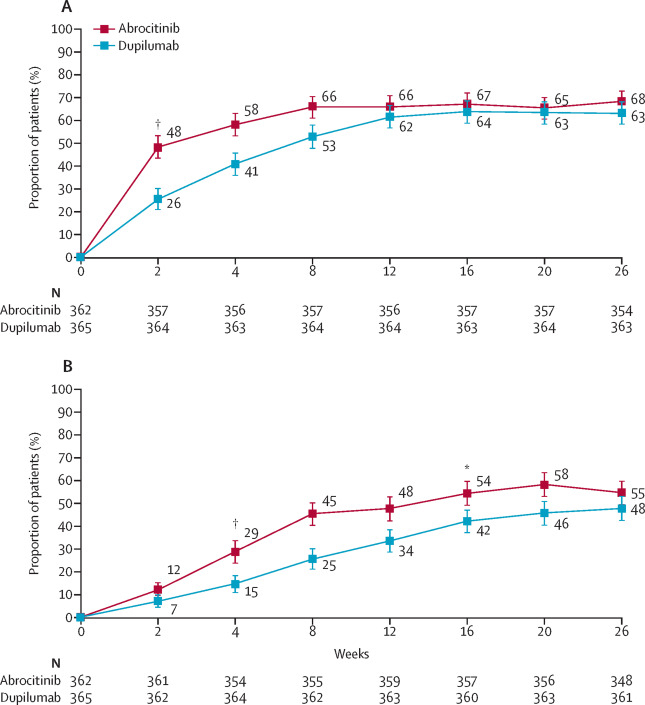
Dupixent relieves the irritation associated with eczema in adults and adolescents within 2 to 4 weeks, and within 16 weeks, the state of the skin has significantly improved.
Dupixent reduced itching in studies on children with severe eczema aged 6 to 11 years old 4.5 times more frequently than a placebo (an inactive medicine). Additionally, twice as many kids on Dupixent experienced skin improvement with clear or almost clear skin.
For people with asthma, there was some improvement in lung function after two weeks, which became noticeable after 12 weeks and persisted for 52 weeks. Up to 81% less severe respiratory exacerbations occurred. About 86% of individuals cut back on or completely stop using oral steroids.
Adults with uncontrolled chronic rhinosinusitis and nasal polyps improved their sense of smell in as little as two weeks. Furthermore, in 24-week and 52-week clinical trials, over 80% fewer patients required nasal polyp surgery.
According to the manufacturer, it takes 16 weeks of Dupixent treatment to achieve steady-state levels.
The Lancet 
Medpage Today -
Medicines that interact with Dupixent may reduce its effect, shorten its duration of action, increase side effects, or have no effect when combined. An interaction between two medications does not always necessitate the discontinuation of one of them; however, it can. Consult your doctor about how to handle drug interactions.
Among the medications that may interact with Dupixent are:
- etanercept
- herbals, such as brewers yeast
- immunosuppressants (such as azathioprine, cyclosporine, or tacrolimus)
- live vaccines and some other vaccines, such as BCG, cholera, measles, or hepatitis b vaccines
- medications used to treat multiple sclerosis, such as fingolimod
- other biologics, such as adalimumab, golimumab, or infliximab
- probiotics, such as bifidobacterium lactobacillus
- warfarin
- zinc.
Any drug that is metabolized by CYP450 enzymes, especially those with a restricted therapeutic index (such cyclosporine or warfarin), carries the risk of interfering with Dupixent. This is due to the possibility that elevated amounts of specific cytokines (like IL-13) during chronic inflammation may affect how CYP450 enzymes are formed. Dupixent prevents IL-4 and IL-13 from interacting with their receptors, which may restore normal CYP450 enzyme synthesis. Keep an eye out for any change in the effect and think about changing the dosage.
Note that only commonly used drugs that may interact with Dupixent are included in this list, which is not exhaustive. For a comprehensive list of interactions, consult the Dupixent prescription instructions.

MIMS 
HSN