Astatine Isotopes Decay in the Blink of an Eye
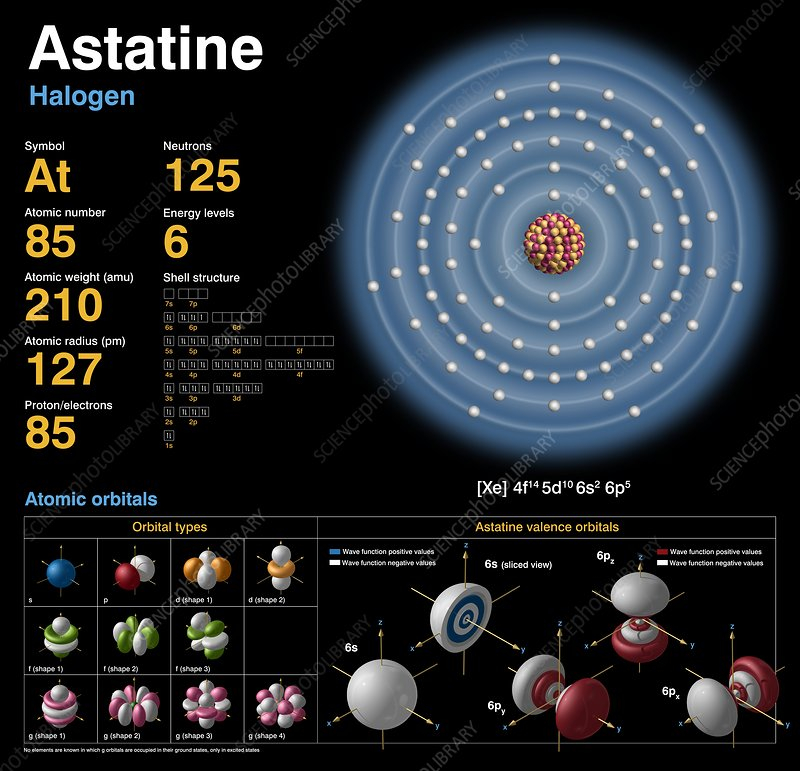
Astatine has a very short half-life compared to tellurium, on the other end of the spectrum. It is the most uncommon element on Earth and is represented by the number 85 on the periodic table. There are only around 25 grams of it on Earth at any given time. Why so uncommon? These half lives are back.
There are 39 radioactive isotopes of astatine (85At), whose mass numbers vary from 191 to 229. All of these isotopes are known. 24 known metastable excited states are also present. The isotope with the longest half-life is 210At, which has an 8.1-hour half-life; the isotope with the shortest half-life in naturally occurring decay chains is 219At, which has a half-life of 56 seconds.
A astatine isotope with a half-life of 8.1 hours is the longest-living one. There are a total of 32 isotopes, none of which are stable. Astatine-213 has the shortest lifetime, measuring in at an astounding 125 nanoseconds. Additionally, it has been asserted that astatine has radioactivity so potent it can actually kill itself.
In a laboratory setting, bismuth may be converted into each of the radioactive isotopes. They occasionally serve as radioactive tracers, but except that, they don't have many other applications in research.