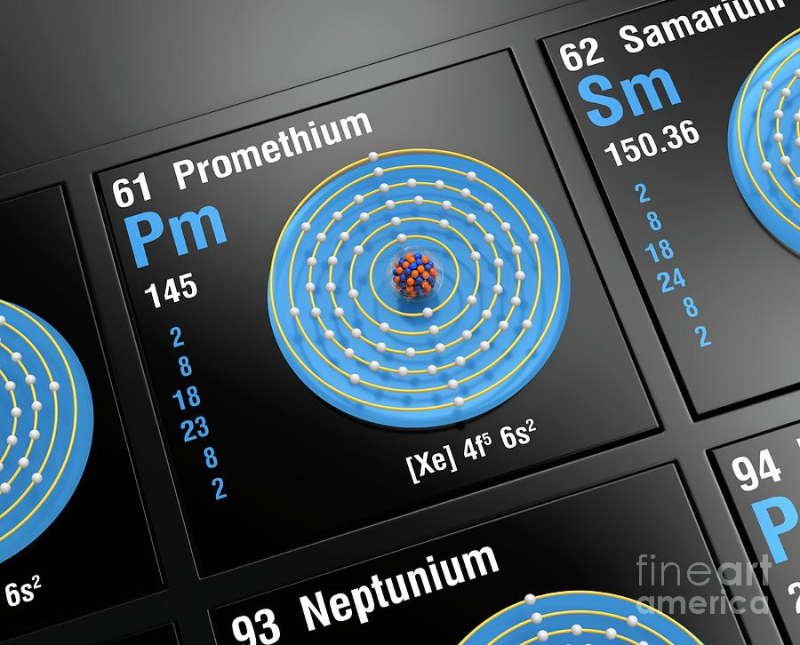
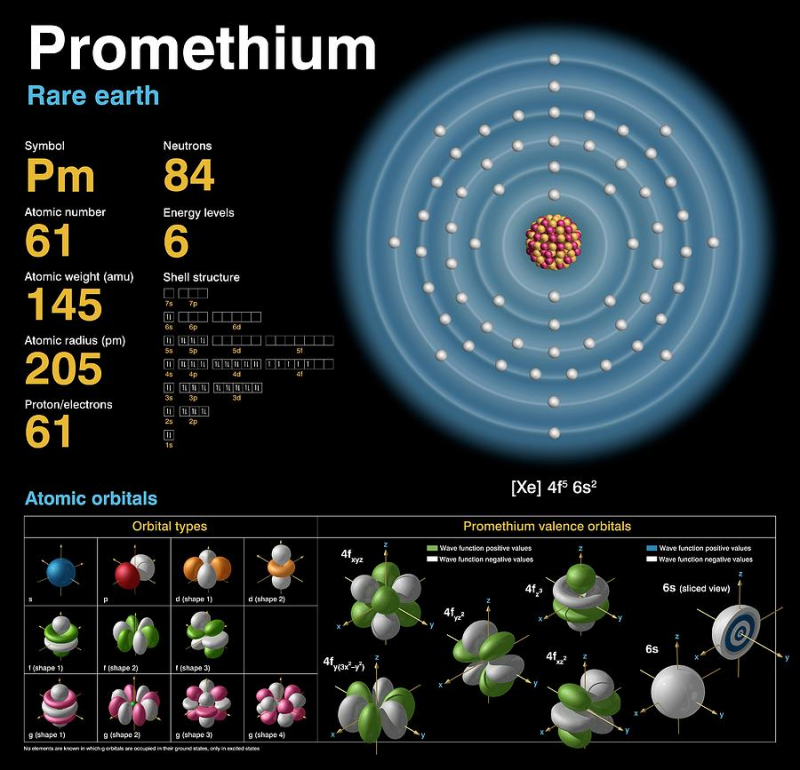
There are Only 500g of Natural Promethium in the World
There are no stable isotopes of the extremely uncommon element promethium, however it does have 38 unstable isotopes. It is incredibly uncommon and emits x-rays. There is currently barely a little over one pound of naturally occurring promethium in the world. However, we can also produce it in a laboratory setting by neutron-beaming uranium-235 and neodymium-147.
Bohuslav Braun, a Czech chemist, predicted that promethium and six other as-yet-unidentified elements would exist in 1902. A few years later, Henry Moseley confirmed that something with the atomic weight of 61 had to exist on the periodic table between neodymium and samarium. After 20 more years of research, it was discovered that element 61, whatever it was, lacked any stable isotopes.
When scientists realized they could artificially create elements and their isotopes, they eventually found promethium after years of searching—but not in nature. You might assume that anything so uncommon and radioactive would have some type of significant application in society at large, but you'd be wrong. In contrast, luminous paint and atomic batteries are the main uses of this material.