Top 10 Most Unusual Isotopes on Earth
Each element's atomic number is unique. The protons in the nuclei are indicated by the atomic number. Isotopes are the same element since they have the same ... read more...number of protons, but they have differing numbers of neutrons. Three isotopes of carbon, such as carbon-12, carbon-13, and carbon-14, exist. Despite having six protons each, they each have six, seven, or eight neutrons. As far as isotopes go, that's rather dull, albeit some of them have some interesting properties.
-
The most prevalent element in the universe is hydrogen, which contains seven isotopes yet only three of them naturally occur. The final one, hydrogen-3, is known as tritium and is both precious and rare. When cosmic rays strike nitrogen in the atmosphere, it can spontaneously develop, but this only results in trace levels. Additionally, it is created as a byproduct of nuclear reactors and in nuclear explosions.
Nuclear weapons are made from tritium, which is also used to make illuminating dials and for a variety of scientific and research endeavors. It is also one of the most expensive materials on earth, pound per pound. For instance, depending on a number of variables, a gram of pure gold may be worth roughly $70. Platinum may cost around $40 per gram.
Depending on where you acquire it, a gram of cocaine might cost as much as $120. A gram of tritium, though? You'll pay roughly $25,000 for that. A nuclear weapon contains about four grams of tritium to boost efficiency, make it more lethal while also making it lighter.

https://blog.banggood.com 
http://allthingsbrass.com/ -
Alkaline earth metal strontium is the 38th element on the periodic table. It has 32 unstable isotopes as well as 4 naturally occurring stable isotopes. You need to keep an eye on strontium-90 because it is the sneakiest of the isotopes.
Due to its high chemical reactivity, strontium-90 can generate heat. Because of this response, it can be utilized as a power source and is employed in the medical sector, remote weather stations, and even spacecraft. The majority of the world's supply comes from nuclear fission, despite the fact that it is not a stable isotope that naturally occurs; nonetheless, it was also created as a result of nuclear weapons tests in the 1950s.
Strontium-90 is a radioactive substance that you should obviously stay away from, but it has a sneaky way of getting you sick if you come into contact with it. Aside from inhalation, it can also enter the body through contaminated food and drink consumption. Once inside, it will be broken down similarly to how calcium is. This implies that the radioactive strontium will ingest into your bones and teeth. It can cause bone cancer, marrow cancer, and soft tissue cancer in the regions close to the radioactive components once it becomes a part of you.

https://de.luciafontains.com/obrazovanie/ 
http://thisisnofantasy.com -
There are no stable isotopes of the extremely uncommon element promethium, however it does have 38 unstable isotopes. It is incredibly uncommon and emits x-rays. There is currently barely a little over one pound of naturally occurring promethium in the world. However, we can also produce it in a laboratory setting by neutron-beaming uranium-235 and neodymium-147.
Bohuslav Braun, a Czech chemist, predicted that promethium and six other as-yet-unidentified elements would exist in 1902. A few years later, Henry Moseley confirmed that something with the atomic weight of 61 had to exist on the periodic table between neodymium and samarium. After 20 more years of research, it was discovered that element 61, whatever it was, lacked any stable isotopes.
When scientists realized they could artificially create elements and their isotopes, they eventually found promethium after years of searching—but not in nature. You might assume that anything so uncommon and radioactive would have some type of significant application in society at large, but you'd be wrong. In contrast, luminous paint and atomic batteries are the main uses of this material.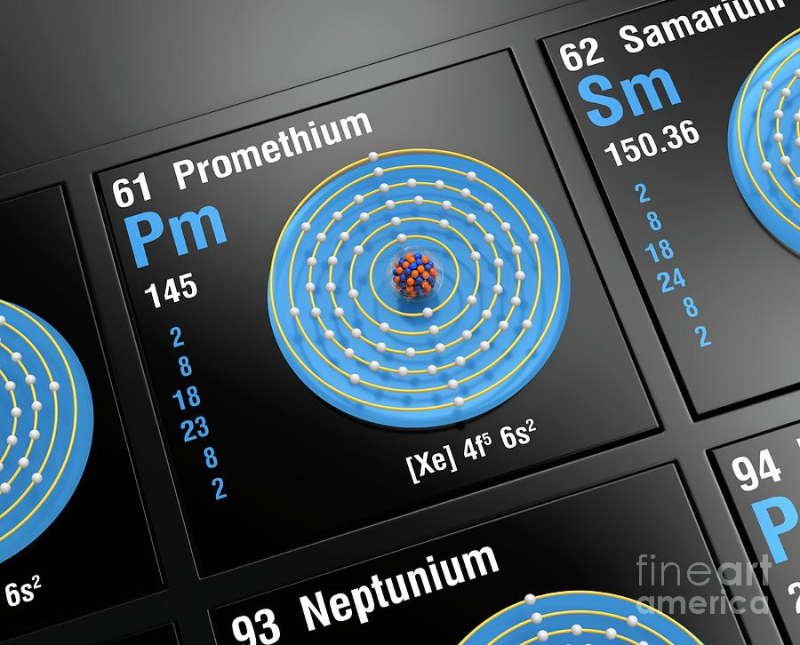
https://fineartamerica.com 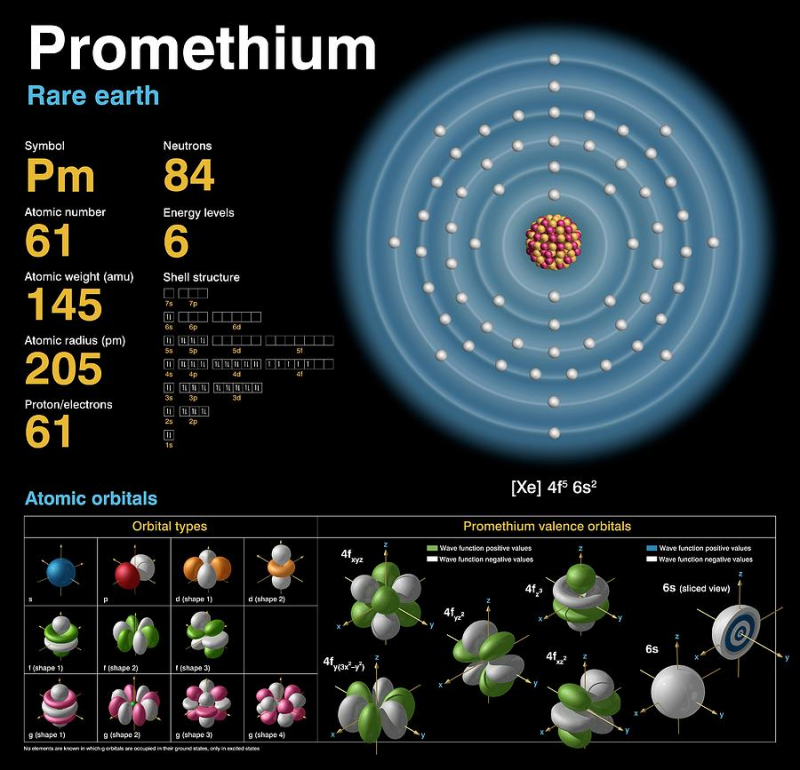
https://fineartamerica.com -
As we've seen, astatine has a very short half life, but researchers have produced a magnesium isotope that is so unstable that they can hardly detect it. Three stable isotopes and 19 unstable ones make up magnesium in its natural state.
Magnesium-18 and magnesium-19 are two of the unstable ones that are particularly intriguing due to their relatively brief lifetimes. The half-life of magnesium-19 is 5 picoseconds. Five trillionths of a second is that. Prepare for magnesium-18 if that sounds like a short period of time, which it is.
Because magnesium-18 only lasts for roughly a sextillionth of a second, it has not been measured sufficiently. It disintegrates so swiftly that it isn't even able to organize electrons around itself to form an actual atom. It just consists of a nucleus before disintegrating. Therefore, scientists can only watch what it did throughout its brief existence rather than studying it directly.

https://horse-direkt.de 
https://periodictable101.blogspot.com/ -
Similar to iron-60, plutonium-244 is not the kind of isotope that appears out of thin air. There aren't any isotopes of plutonium that, unlike iron, you really want to get too close to. There are a total of 20 of them, and while they are all radioactive, plutonium-244 is the most stable.
In 2021, plutonium-244 traces were discovered in the ocean floor, and it is thought that they traveled a long way to get there. Plutonium-244 is produced under some extremely demanding conditions. In this instance, two stars collided and caused a significant explosion, which produced the plutonium. Along with iron-60, it most likely also develops in supernovae.
Plutonium-244 is not at all an isotope that can be produced easily on Earth. Nuclear reactors produce a variety of isotopes, but plutonium-242 only has a short half-life as 243 and cannot further decay to plutonium-244 due to this. Nuclear weapon explosions have been predicted to create 244, however this is simply a prediction and has not been verified.

https://www.zazzle.com/ 
https://www.istockphoto.com -
Everyone is familiar with iron, one of the most widespread elements in the world that is so significant that an entire era is named after it. Without iron, all of our magnets would break, not to mention that we couldn't produce nearly anything else in the world that is made of metal. There is a ton of crude iron ore in the world, with an estimated 800 billion tons of it containing 230 billion tons of iron.
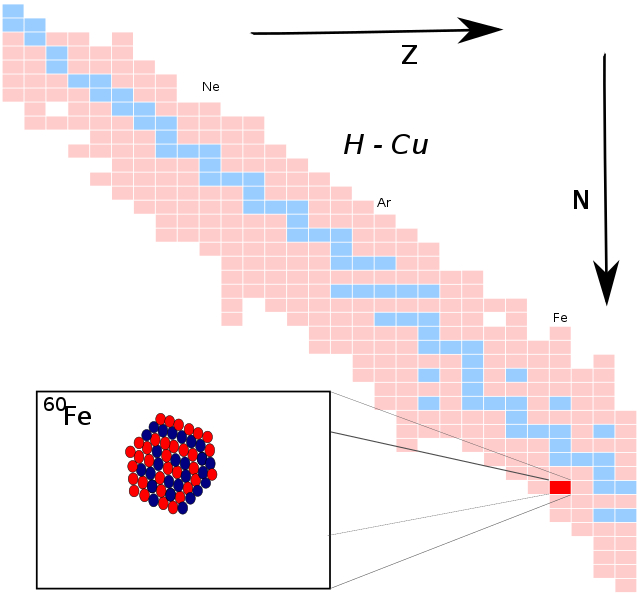
There are 24 radioactive isotopes and four stable isotopes of iron. The most stable of them, iron-60, has a half-life of about 2.6 million years. Additionally, it's not the kind of thing that appears randomly. You'll need to search for iron-60 if you want to have your own because it is created during star explosions, such as supernovae.
Iron-60 is ejected into space when stars explode, and trace amounts of it occasionally find their way to Earth. Even some of it has been found in the snow of Antarctica. Five iron-60 atoms were discovered in the 500 kilograms of pristine snow that were gathered to find samples. Only 23 years have passed since the isotope was found for the first time on Earth in some deep-sea soil layers.
https://commons.wikimedia.org 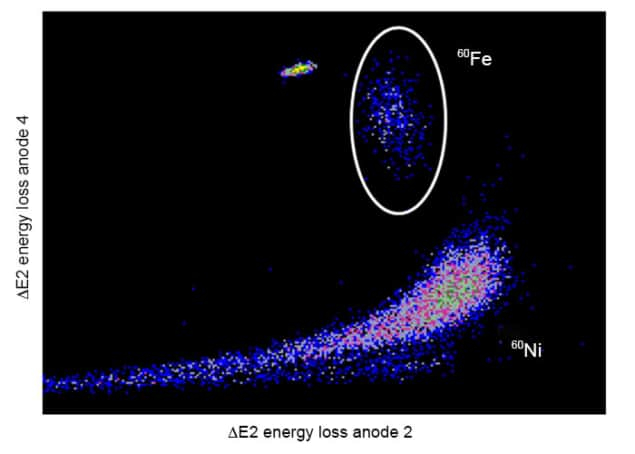
http://physicsworld.com/ -
Another metalloid that lies on the border between metal and non-metal, germanium is the 32nd element in the periodic table. Germanium-76 is the longest-living of its five stable isotopes, having a half-life that is around 130 billion times longer than the age of the universe. However, that is not the most fascinating isotope. When Germanium begins to warm up, isotope 72 exhibits a highly peculiar property.
We know that when heat is introduced to a substance, certain things happen to the atoms. Heat causes the atoms to become excited and move more quickly, similar to how it does with substances like water. What transpires, though, inside the atoms themselves? It's not as clear as you might assume, as Germanium-72 demonstrates.
In germanium, there are 32 protons, while in germanium-72, there are 40 neutrons. As the atom warms up, the strong pairs formed by those protons become weaker. With our water example in mind, that makes sense. The issue is that eventually, something strange occurs. The link between proton pairs really strengthens once again at high enough temperatures. This is due to a phenomenon known as a phase transition. As the transition gets going, things stabilize before they start to weaken again as the temperature continues to climb.

https://www.buyisotope.com/ 
https://nukem-isotopes.de -
Most people would be thrilled to find some gold in the nature because it is a fairly well-known element. In contrast to many other metals, it doesn't corrode and is quite valuable. There are actually 41 known isotopes of the metal, which is something that is less generally recognized. The only stable isotope of gold that humans are aware of and desire is gold-197. The remaining forty are all radioactive.
Interesting stability is observed with gold-197. This indicates that although science predicts it should be radioactive, observation contradicts this prediction. So, unlike what you may assume, it just isn't radioactive. That has generally benefited practically every economy throughout history.
By using half-lives, we can gauge radioactivity in one way or another. The length of time it takes for half of a sample of a radioactive isotope to experience a nuclear decay event is known as the radioactive half-life. If a radioactive substance has a sample with a half-life of one day, just half of your sample will still contain the original substance after that time. The remaining portion could transform into several isotopes of the same element or atoms of a different element.

https://gainspainscapital.com/ 
https://www.goldmoney.com/ -
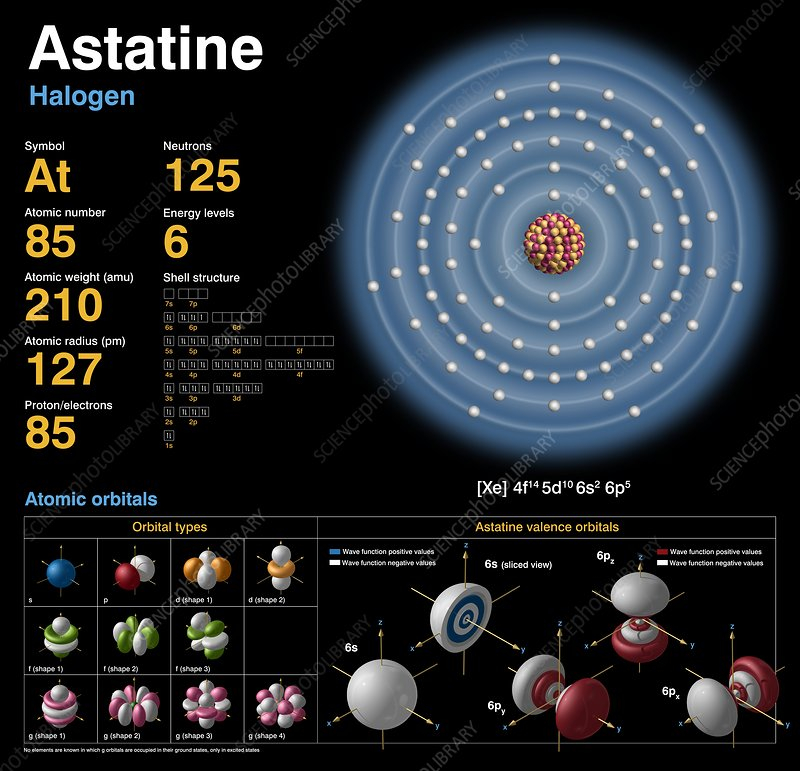
Astatine has a very short half-life compared to tellurium, on the other end of the spectrum. It is the most uncommon element on Earth and is represented by the number 85 on the periodic table. There are only around 25 grams of it on Earth at any given time. Why so uncommon? These half lives are back.
There are 39 radioactive isotopes of astatine (85At), whose mass numbers vary from 191 to 229. All of these isotopes are known. 24 known metastable excited states are also present. The isotope with the longest half-life is 210At, which has an 8.1-hour half-life; the isotope with the shortest half-life in naturally occurring decay chains is 219At, which has a half-life of 56 seconds.
A astatine isotope with a half-life of 8.1 hours is the longest-living one. There are a total of 32 isotopes, none of which are stable. Astatine-213 has the shortest lifetime, measuring in at an astounding 125 nanoseconds. Additionally, it has been asserted that astatine has radioactivity so potent it can actually kill itself.
In a laboratory setting, bismuth may be converted into each of the radioactive isotopes. They occasionally serve as radioactive tracers, but except that, they don't have many other applications in research.
https://www.sciencephoto.com/ 
https://www.ionetix.com/ -
On the periodic table, tellurium is a silvery metalloid element at position 52. Try not to play with it because it's a little harmful as well. It has eight isotopes, and tellurium-128, one of them, set a Guinness World Record for having the longest half-life ever observed.
Calculated at 2.2 x (10 to the power of 24) years, the number. You can also say it another way, as it is a number that most of us find difficult to pronounce. It has existed for 160 trillion trillion years. Since the cosmos is already about 14 billion years old, tellurium-128 has a half-life that is as close to infinity as you're going to find in nature.
Although it has been asserted that 123Te electron capture has been observed, this has been refuted by the same team's most recent results. 123Te has a half-life that is greater than 9.2 1016 years and perhaps considerably greater. Te can be used as a starting material in particle accelerators like cyclotrons to create radionuclides. Iodine-123 and iodine-124 are two typical radionuclides that can be created from tellurium-124.

https://images-of-elements.com/ 
http://images-of-elements.com/






























