Top 7 Things to Know About Cosentyx
Cosentyx is an antibody that blocks a specific protein in the body that can cause inflammation. So it inhibits and blocks the activity that causes inflammation ... read more...and swelling. In this article, Toplist gives you everything you need to know about this drug.
-
Cosentyx is the brand name (trade name) for secukinumab, a medication used to treat plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and other conditions.
Secukinumab (Cosentyx) works by selectively binding to the pro-inflammatory cytokine interleukin 17A (IL-17A), which is produced by T-helper cells. Innate immunity (natural immunity) to infecting substances is mediated by IL-17A, and it can also contribute to the development of chronic inflammatory diseases such as psoriasis and ankylosing spondylitis.
Secukinumab inhibits the interaction of IL-17A with the IL-17 receptor, thereby preventing inflammation.
Cosentyx belongs to the class of drugs known as interleukin inhibitors. It is also known as a human IgG1 monoclonal antibody.

Verywell Health qmqmx qmqmx -
Cosentyx may be used to treat adults and children over the age of six with moderate to severe plaque psoriasis who require systemic therapy or phototherapy.
Cosentyx may also be used to treat active psoriatic arthritis in adults, adolescents, and children aged 2 and up, as well as enthesitis-related arthritis in adults and children aged 4 and up.
Adults with active ankylosing spondylitis and active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation may also benefit from Cosentyx. Cosentyx may be used for conditions other than rheumatoid arthritis.
Cosentyx reduces inflammation by inhibiting the release of proinflammatory cytokines and chemokines.
Cosentyx is usually given once a month by subcutaneous injection after an initial loading dose (which for plaque psoriasis consists of 300mg doses of Cosentyx given every week for five doses) (under the skin). Cosentyx is typically administered in 300mg doses (two injections), but some patients may only require a 150mg dose.
Cosentyx is typically administered in 150mg doses, with or without loading doses, for other conditions. If there is no response, higher doses may be administered.
Cosentyx comes in three forms: a Sensoready pen, a prefilled syringe, and lyophilized powder in a vial for reconstitution. The lyophilized powder for reconstitution is only for use by healthcare providers.
A healthcare professional can teach people who have been prescribed Cosentyx how to self-administer the Sensoready pen or prefilled syringe.
Although Cosentyx has few side effects, it may increase the risk of infection.
Cosentyx 
COSENTYX® (secukinumab) -
If you are between the ages of 18 and 60, do not take any other medications, and have no other medical conditions, you are more likely to experience the following side effects:
- The most common side effect reported with Cosentyx is nasopharyngitis, which affects 11%-12% of people. Diarrhea, upper respiratory tract infection, rhinitis, oral herpes, and pharyngitis are among the 1% to 5% of people who experience side effects.
- Pain, redness, and swelling at the injection site, nausea, neutropenia (low levels of a type of white blood cell), thrombocytopenia (low platelet levels), and other infections are also common. Urticaria is a possibility.
- Cosentyx increases the likelihood of infection because it affects the immune system, reducing its ability to respond to pathogens. Upper respiratory tract infections, conjunctivitis, tinea, and oral candidiasis are the most common infections reported, and the rate of infection for Cosentyx in one trial was 28.7% compared to 18.9% of subjects treated with a placebo (an inactive treatment). Serious infections occurred in 0.14% of Cosentyx-treated patients versus 0.3% of placebo-treated patients. Cosentyx should not be started in people who have infection symptoms, and your doctor may decide to discontinue Cosentyx, either temporarily or permanently, if you develop an infection while taking Cosentyx.
- Before administering Cosentyx, all patients should have current vaccinations. Do not give live vaccines to people taking Cosentyx. Non-live vaccinations received during a course of Cosentyx may not elicit an immune response strong enough to prevent disease.
- Before you start Cosentyx, your doctor will ask if you have ever been exposed to tuberculosis (TB). If their investigations reveal that you have been exposed to tuberculosis, they may conduct additional tests and require you to take antibiotics. If you develop TB symptoms (such as a persistent cough that lasts more than three weeks, chest pain, or coughing up blood or sputum), contact your doctor right away.
- Serious hypersensitivity reactions (such as angioedema and urticaria) have been reported in a small number of Cosentyx patients. The first dose of Cosentyx should always be administered by a healthcare professional, and patients should be advised to seek immediate emergency care if they experience an allergic reaction. People who have previously had an allergic reaction to Cosentyx or any of its excipients should not be given Cosentyx.
- Patients taking Cosentyx are more likely to develop inflammatory bowel disease. Diarrhea, abdominal pain, fever, blood in the stool, and weight loss are all possible symptoms.
- Cosentyx is only available as an injection given through the skin. Before use, store it in the refrigerator at 2ºC to 8ºC (36ºF to 46ºF). Cosentyx does not contain preservatives, and the manufacturer recommends that the pre-filled syringe or SensoReady pen be discarded if left unrefrigerated for more than 4 days at room temperature, not exceeding 30°C. If Cosentyx has been left out of the fridge for more than four days, discard it. Cosentyx contains no preservatives, and microbial contamination may occur if stored improperly.
- The removable cap of the Cosentyx Sensoready pen and the Cosentyx prefilled syringe contain natural rubber latex. If latex-sensitive people handle the cap, they may develop an allergic reaction.
- It is unknown what effect Cosentyx has on the unborn child during pregnancy. There is no information on the effect of Cosentyx on lactation.
Seniors and children, people with certain medical conditions (such as liver or kidney problems, heart disease, diabetes, seizures), or people who take other medications are more likely to experience a broader range of side effects.
Everyday Health 
Sidra Medicine -
Cosentyx is a prescription medication that is used to treat plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and other conditions. Cosentyx must be injected subcutaneously (under the skin) every four weeks after an initial loading dose, but most people can be taught how to self-administer either the Sensoready pen or prefilled syringe. The most commonly reported side effects are nasopharyngitis and an increased risk of infection.
Cosentyx 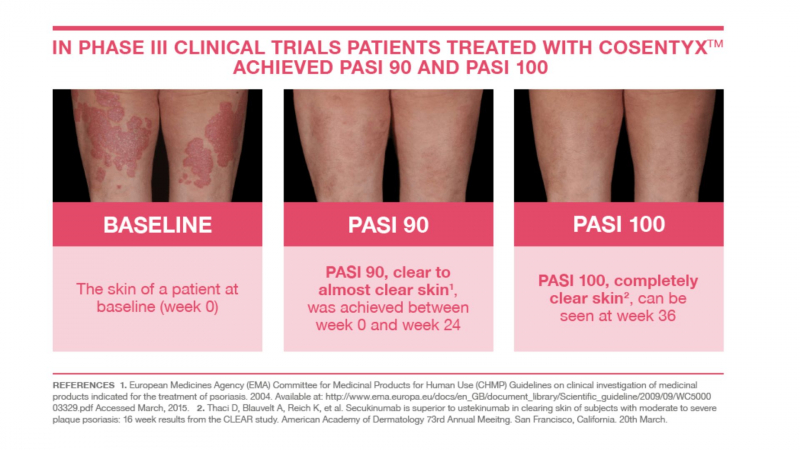
SkinCare Physicians -
If you have been shown how to self-inject Cosentyx, give yourself a dose once a month, preferably on the same day of the month; otherwise, see your healthcare provider once a month for a dose. If you forget a dose, schedule it as soon as possible, then resume your regular dosing schedule. If you have not been shown how to inject Cosentyx by a health professional, do not attempt to do so.
Take one or two (depending on whether your dose is 150mg or 300mg) Cosentyx injections from the refrigerator and place them on a flat surface, away from children and pets, to warm up to room temperature for 15 to 30 minutes, no longer. Warm up in no other way (such as by putting in hot water). At room temperature, Cosentyx reduces the risk of stinging. If the liquid is cloudy, contains particles, or is discolored, inspect the injection for discoloration or particulate matter and discard it. Cosentyx liquid should be clear, colorless, or have a slight yellow tinge. Check the expiry date on the injection's side and do not use Cosentyx if it has passed. Cosentyx should not be shaken.
Cosentyx is best injected into the front of your thighs or lower abdomen, avoiding the area around your belly button (stay and inch away from your belly button). If someone else is administering your injection, they can also inject it into the outer area of your upper arm. Change your injection site once a month to avoid injecting into the same spot every time.
Inject only into clear, healthy skin. Do not inject into any bruised, tender, red, scaly, or hard areas. You should also avoid injecting into scars, stretch marks, or psoriasis-affected areas.
If you experience pain when injecting Cosentyx, applying an ice pack to the area of skin where you inject Cosentyx for a few minutes before and after the injection can help.
You should not shake the Cosentyx Sensoready pen before using it. After washing your hands, wipe the injection site with an alcohol wipe and allow it to dry. Remove and discard the cap. Hold the pen at a 90-degree angle (straight up and down) to your preferred injection site and press firmly against your skin with your fist. The pen will make two clicks. The first click indicates that the injection has begun, and you must keep the pen firmly in place. The second click indicates that the injection is nearly complete, but you must continue to hold the pen in place until all of the medicine has been dispensed. When the green indicator fills the window and stops moving, the medication has finished dispensing. Take the pen out of your skin. Place the used pen in a sharps container approved by the FDA. Cosentyx Sensoready Pens each contain 150mg of Cosentyx. If you are taking 150mg Cosentyx, use one Cosentyx Sensoready Pen; if you are taking 300mg Cosentyx, use two pens (one at a time) and repeat the above steps for the second pen.
If you're using a prefilled Cosentyx syringe, wash your hands first, then wipe the injection site with an alcohol wipe and let it dry. Throw away the needle cap from the Cosentyx prefilled syringe. Hold the prefilled syringe in one hand and gently pinch the skin at the injection site with the other to form a small bump of skin. Then, at a 45-degree angle, insert the needle into the small bump, making sure to push it all the way in. Place your first two fingers on the syringe's finger grips and slowly push down the plunger as far as it will go with your thumb. Once fully depressed (the plunger head should end between the syringe guard wings), hold the injection for 5 seconds to ensure that all of the medicine has been injected. You can now remove the injection from your skin while keeping the plunger pushed in. Once the injection has been removed from the skin, the plunger can be slowly released, allowing the needle to be covered by the syringe guard. Use an FDA-approved sharps container to dispose of the used Cosentyx prefilled syringe.
Cosentyx should be kept in the refrigerator. Try to use it within one hour of taking it out of the refrigerator. If you inadvertently leave it out of the refrigerator for an extended period of time, the manufacturer recommends that the pre-filled syringe or SensoReady pen be stored at room temperature for up to 4 days, not exceeding 30°C. If Cosentyx has been left out of the fridge for more than four days, discard it. Cosentyx contains no preservatives, and microbial contamination may occur if stored improperly. Cosentyx should not be frozen. Keep light away from Cosentyx.
If you experience shortness of breath, facial or throat tightness after taking Cosentyx, seek immediate medical attention. Inform your doctor right away if you feel ill or notice other signs of infection. Remember to notify your doctor if you experience any other side effects, such as dizziness or fainting.
Cosentyx is linked to an increased risk of developing inflammatory bowel disease. Any symptoms such as diarrhea, abdominal pain, fever, blood in the stool, or weight loss should be reported to your doctor.
Inform other medical professionals that you are receiving Cosentyx. All of your vaccinations should be up to date before starting Cosentyx. You should not receive any live vaccines while taking Cosentyx (such as the MMR vaccine or the chickenpox vaccine).
Cosentyx is not recommended during pregnancy unless the benefits outweigh the risks; however, if you become pregnant while taking Cosentyx, notify your doctor immediately.
Before taking any other medications with Cosentyx, consult your doctor or pharmacist.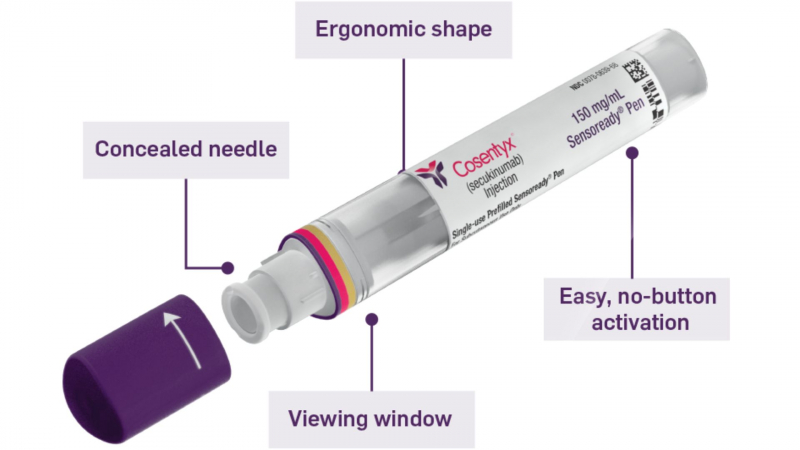
Cosentyx 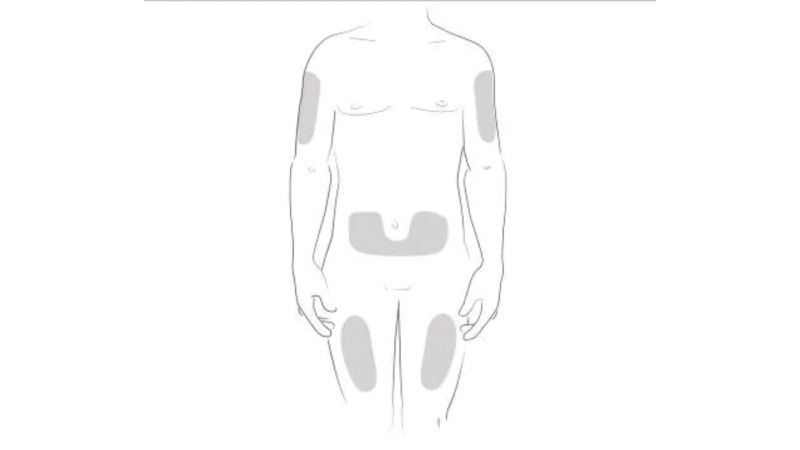
Medicine.com -
Cosentyx begins to work within a few weeks, but full effects may not be seen for up to four months.
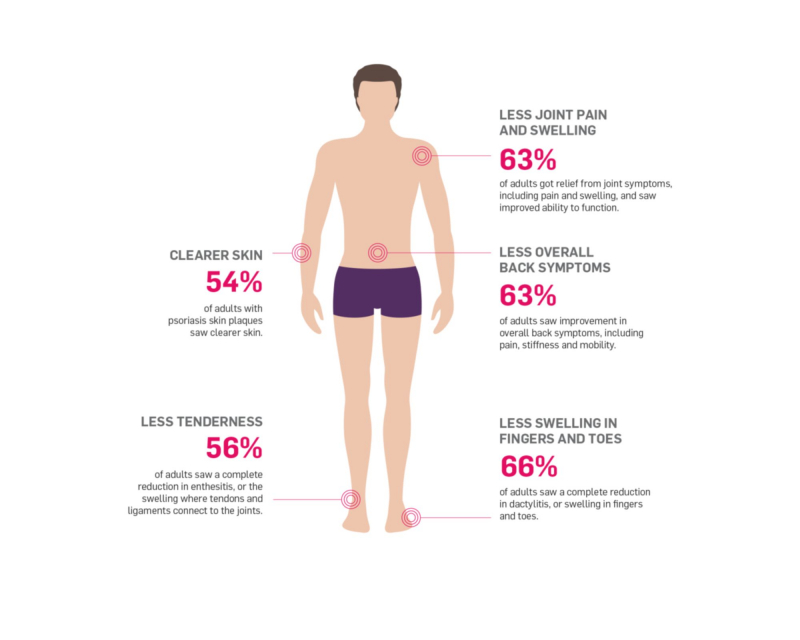
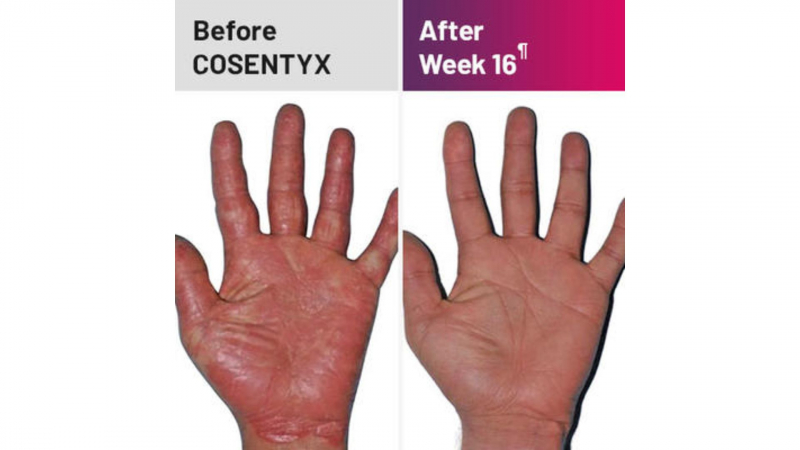
After 12 weeks of taking Cosentyx 300mg, 77.1% of patients had a 75% reduction in their PASI (Psoriasis Area and Severity Index) score. After 16 weeks, this figure had risen to 86.7% of patients.
After 4 weeks, 50% of patients with psoriatic arthritis had an ACR 20 (this corresponds to a 20% improvement in psoriatic arthritis). By 16 weeks, 62.6% of psoriatic arthritis patients had reached ARC20, and 39.6% of patients had reached ARC50.
The FUTURE 5 study found that 91.8% of psoriatic arthritis patients taking Cosentyx 300mg experienced no radiographic disease progression over the course of 52 weeks.
It took 3 weeks for 50% of Ankylosing Spondylitis (AS) patients to achieve an ASAS20 (20% improvement in AS). At 3 weeks, the ASAS40 response was more than 20%, and by 16 weeks, the number of patients with an ASAS20 response had increased to 61.1%, while the number of patients with an ASAS40 response was 36.1%.
X-rays revealed that 79% of AS patients on long-term Cosentyx 150 mg therapy had no definite disease progression in the MEASURE 1 study.
By 16 weeks, 13% of patients with non-radiographic Axial Spondyloarthritis had a response of ASAS40 (40% improvement), which increased to 19% by week 52.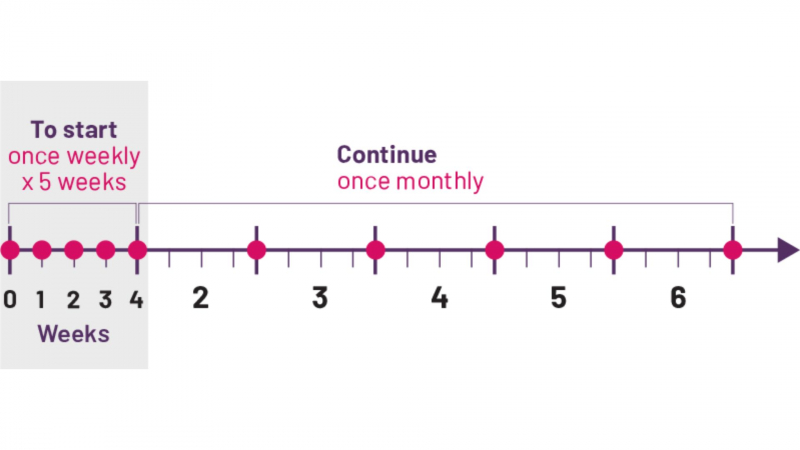
Cosentyx 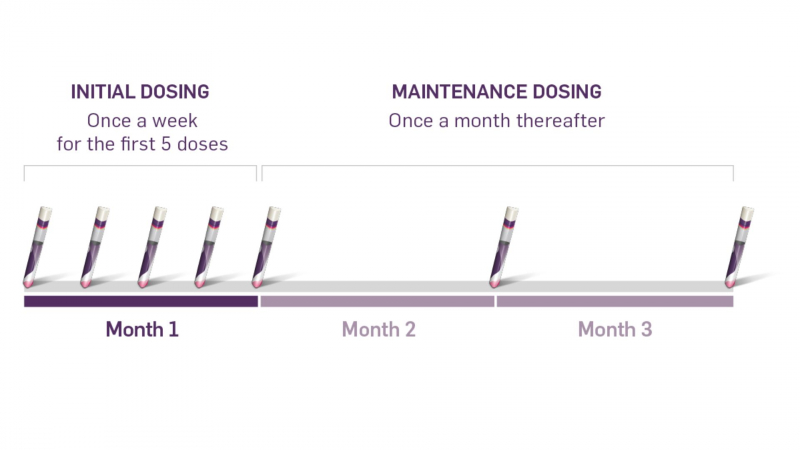
COSENTYX -
Medicines that interact with Cosentyx may reduce its effect, shorten its duration of action, increase side effects, or have no effect when combined. An interaction between two medications does not always necessitate the discontinuation of one of them; however, it can. Consult your doctor about how to handle drug interactions.
Cosentyx may interact with the following medications:
- aminophylline
- anticonvulsants, such as phenobarbital,
- benzodiazepines, such as alprazolam or clonazepam
- chemotherapy agents, such as cyclophosphamide, methotrexate, or bleomycin
- corticosteroids (such as prednisone or dexamethasone)
- cyclosporine
- heart medications such as amiodarone or flecainide
- herbals, such as echinacea
- hormones such as levonorgestrel or norgestrel
- immunosuppressants (such as azathioprine, cyclosporine, or tacrolimus)
- live vaccines and some other non-live vaccines, such as BCG, cholera, measles, or hepatitis b vaccines
- opioids, such as fentanyl or oxycodone
- other biologics, such as adalimumab, golimumab, or infliximab
- probiotics, such as lactobacillus
- statins, such as atorvastatin or simvastatin
- theophylline
- warfarin
- zinc.
Any medication that is metabolized by CYP450 enzymes, particularly those with a narrow therapeutic index (such as cyclosporine or warfarin), has the potential to interact with Cosentyx. This is because increased levels of certain cytokines (such as IL-1, IL-6, IL-10, and IL-17) during chronic inflammation can alter the formation of CYP450 enzymes. Because Cosentyx prevents IL-17A from interacting with the IL-17 receptor, it may restore CYP450 enzyme formation. Keep an eye out for any changes and consider changing the dosage if necessary.
It should be noted that this list is not exhaustive and only includes common medications that may interact with Cosentyx. For a complete list of interactions, consult the Cosentyx prescribing information.
Medicover Hospitals 
NPS MedicineWise