Top 8 Things to Know About Invokana
With so many medication choices available, Type 2 diabetics may feel overwhelmed. Various medications, including sodium glucose co-transporter 2 (SGLT2) ... read more...inhibitors, are available to manage diabetes. The FDA authorized Invokana (canagliflozin) in 2013 as the first SGLT2 inhibitor. But what are the purposes of invokana and what should you know before using it? In the list below, we'll go over 10 things you should know about Invokana, such as how it functions, who can use it, and potential side effects.
-
Invokana is only approved by the FDA for people with Type 2 diabetes. When combined with a healthy diet and exercise, it can help lower your blood sugar.
However, Invokana has other applications. It has been shown to reduce the risk of certain people's heart problems worsening. Invokana has been approved by the Food and Drug Administration to reduce the risk of heart attack, stroke, and even death in people with Type 2 diabetes and heart disease.
Invokana can help people whose kidney disease is caused by diabetes (diabetic nephropathy) and protein in their urine from getting worse. Invokana is FDA-approved for people with this type of kidney disease to reduce the risk of:
- Worsening kidney disease
- Death due to heart problems
- Being hospitalized for heart failure
Even though Invokana is only FDA-approved for people with Type 2 diabetes, the American College of Cardiology and American Heart Association both recommend an SGLT2 inhibitor for some people with heart failure, regardless of whether they have diabetes. This is an off-label use of Invokana, but it is supported by substantial clinical evidence.
Photo by Diabetesmagazijn.nl on Unsplash 
Photo by Nataliya Vaitkevich on Pexels: https://www.pexels.com/photo/a-letter-dice-near-glucose-meter-and-insulins-6941880/ -
Invokana is available in 100 mg and 300 mg tablet forms. The recommended starting dose is 100 mg before your first meal of the day.
During treatment, your healthcare provider will monitor your lab results. They'll check to see if your kidneys are working properly. They'll also check to see if Invokana has reduced your blood sugar. If your kidneys are functioning normally but your blood sugar remains high, your doctor may increase your dose. The maximum daily dose is 300 mg.

Photo by Rūdolfs Klintsons on Pexels: https://www.pexels.com/photo/grayscale-photo-of-medicines-and-drugs-7380393/ 
Photo by Towfiqu barbhuiya on Pexels: https://www.pexels.com/photo/man-pointing-on-drugs-on-his-hand-8846504/ -
A hormone called insulin isn't working properly or you're not producing enough of it if you have Type 2 diabetes. Your body uses insulin to metabolize glucose (sugar). Insufficient insulin can cause blood glucose levels to rise. If their glucose levels continue to be elevated, people with diabetes can experience a variety of health issues.
As was already stated, Invokana belongs to a class of drugs known as SGLT2 inhibitors. Invokana (Canagliflozin) functions by preventing the sodium-glucose co-transporter 2 (SGLT2) from carrying glucose from the blood that passes through the kidneys (the glomerular filtrate) back into circulation. SGLT2 is a protein found in the early proximal tubule of a nephron or kidney cell. Canagliflozin stops glucose from being reabsorbed and increases the amount of glucose excreted in the urine by blocking this transporter. This lowers blood sugar and raises A1C.
But Invokana does more than just reduce blood sugar levels. Additionally, it stops sodium from returning to circulation. This reduces kidney pressure, which can eventually harm them in higher levels.
Photo by Kate on Unsplash 
Photo by Steve Buissinne on Pixabay -
Metformin is a first-line treatment for Type 2 diabetes. If your blood sugar remains high after taking metformin alone, you can combine it with Invokana. This combination of metformin and Invokana has been shown in studies to effectively lower blood sugar levels. In fact, canagliflozin and metformin are combined in single medications known as Invokamet (canagliflozin/metformin) and Invokamet XR (canagliflozin/metformin extended release).
Invokana has also been investigated as a supplement to other medications such as sulfonylureas and insulin. When combined with other diabetes medications, Invokana helped lower blood sugar levels in these studies. However, if you combine Invokana with insulin or other diabetes medications, your doctor will want to make sure your blood sugar doesn't drop too low.
Invokana can be used on its own in patients who cannot take metformin, despite the fact that it is typically taken in combination with another drug.
Photo by Artem Podrez on Pexels: https://www.pexels.com/photo/food-healthy-art-blue-6823735/ 
Photo by Pavel Danilyuk on Pexels: https://www.pexels.com/photo/a-woman-using-an-insuling-injection-pen-7653660/ -
Medicines that interact with Invokana may reduce its effect, shorten its duration of action, increase side effects, or have no effect when combined. An interaction between two medications does not always necessitate the discontinuation of one of them; however, it can. Consult your doctor about how to handle drug interactions.
The following are examples of medicines that may interact with Invokana:
- acetazolamide
- anticonvulsants such as phenytoin or phenobarbital
- antipsychotics, such as aripiprazole or clozapine
- beta-blockers, such as atenolol, labetalol, and metoprolol, may enhance the hypoglycemic effects
- ciprofloxacin or gatifloxacin
- corticosteroids, such as prednisone or cortisone
- digoxin
- diuretics, such as bumetanide, HCTZ, and bendroflumethiazide, which may enhance the potential for volume depletion
- HIV medications, such as amprenavir, atazanavir, fosamprenavir, and ritonavir
- hormones, such as ethinylestradiol and hydroxyprogesterone
- insulin (may increase risk of hypoglycemia)
- isoniazid
- rifampin
- other medications that affect blood sugar levels or are used for diabetes, such as glimepiride, or metformin.
Since SGLT2 inhibitors like Invokana raise urinary glucose excretion and can cause positive urine glucose tests, it is not recommended to use urine glucose tests to monitor glucose control in patients taking them. It is advisable to use alternative techniques for monitoring glucose management. There may also be interference with 1,5-AG tests.
Note that only commonly used drugs that may combine with Invokana are included in this list, which is not exhaustive. For a comprehensive catalog of interactions, consult the Invokana prescribing information.
Photo by Anna Shvets on Pexels: https://www.pexels.com/photo/different-medication-pills-and-capsules-on-red-background-3683040/ 
Photo by freestocks on Unsplash -
Yes, it is possible. Invokana has been studied in comparison to placebo and a variety of other diabetes medications. These are some examples:
- Metformin alone
- Sulfonylureas combined with metformin
- Dipeptidyl peptidase-4 (DPP-4) inhibitors (gliptins) combined with metformin
- Glitazones combined with metformin
- Insulin alone
In all of these studies, Invokana resulted in greater weight loss than the medication regimen to which it was compared. When Invokana was added to these studies, participants lost 2% to 4% of their body weight.

Photo by Total Shape on Pexels: https://www.pexels.com/photo/scrabble-pieces-on-a-plate-2377045/ 
Photo by i yunmai on Unsplash -
In women, yeast infections are the most common side effect of Invokana. These occur as a result of Invokana increasing the amount of sugar in the urine. This creates an environment in which yeast and bacteria can thrive. Male yeast infections can also be caused by Invokana.
Other common Invokana side effects include:
- Urinary tract infections (UTIs)
- Greater urge to urinate
- Greater thirst
- Constipation
- Nausea
- Vaginal itching

Photo by Kyle Glenn on Unsplash 
Photo by Alexa on Pixabay -
Invokana can have more severe side effects, according to some research.
Invokana has been associated with:
- Higher risk of leg and foot amputations
- Extreme fluid loss
- Ketoacidosis, a serious issue of diabetes where blood sugar is dangerously high
- Serious kidney infections
- Necrotizing fasciitis (also called “flesh-eating bacteria”) in the genital area
- Bone fractures
- Low blood sugar (hypoglycemia) when used with certain medications
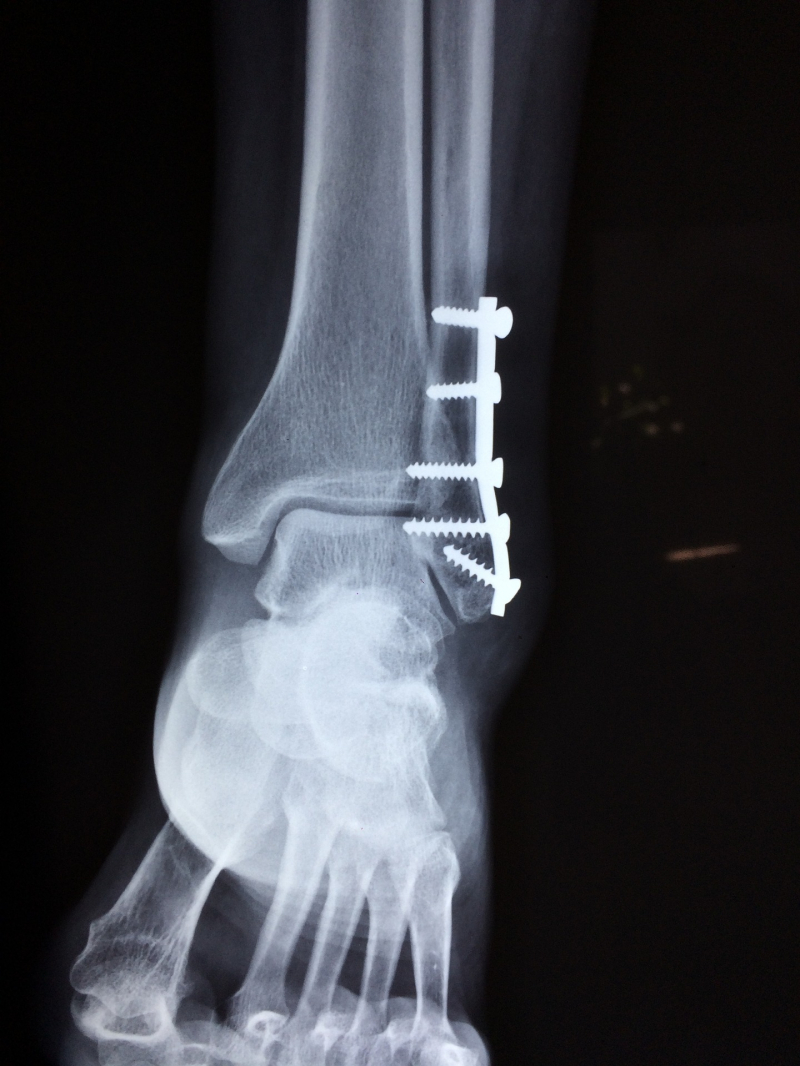
Photo by Dr. Manuel González Reyes on Pixabay 
Photo by Nataliya Vaitkevich on Pexels: https://www.pexels.com/photo/person-holding-black-and-white-electronic-device-6940861/










